Exploring Blood-Based Biosurveillance, Part 2: Sampling Strategies within the US Blood Supply
By ljusten, harmonbhasin @ 2024-09-10T16:39 (+14)
This is a linkpost to https://naobservatory.org/blog/exploring-blood-biosurveillance-part2
This is the second post in a series of blogs exploring blood-based biosurveillance for novel pathogen detection as part of the NAO’s effort to evaluate different biosurveillance approaches. We once again thank our colleagues at the NAO for their valuable feedback. All mistakes are our own.
Summary
- The U.S. blood supply system, encompassing both transfusion medicine and the plasma industry, offers a promising foundation for large-scale, pathogen-agnostic biosurveillance due to its large donor base and established infrastructure.
- Our analysis identifies several promising sampling strategies: sampling from individual whole blood or source plasma testing aliquots, whole blood ‘mini-pools,’ and plasma manufacturing pools.
- Among the identified strategies, partnering with large non-profit organizations like the American Red Cross or regional blood centers to access whole blood samples appears most promising. These samples are available soon after donation and, being whole blood, may enable detection of intracellular pathogens.
- This post does not examine all potential blood sampling strategies, only those related to the transfusion medicine field and the plasma industry. Other samples not covered here include diagnostic lab discards, blood biobanks, and direct volunteer sampling.
Introduction
In our previous post, we examined the physical and biological characteristics of blood to determine its viability as a sample type for pathogen-agnostic biosurveillance. These characteristics suggested that metagenomic sequencing of human whole blood or plasma could detect an important subset of emerging pandemic threats. Indeed, previous research has demonstrated the detection of novel pathogens in blood (1–3).
However, past efforts to use blood for novel pathogen detection have largely been limited to one-off scientific investigations, neglecting to explore the design of an ongoing, effective blood sampling and surveillance system. This post aims to bridge the gap between these scientific studies of the human blood virome and practical sampling strategies that leverage the existing blood supply for continuous pathogen surveillance.
Our analysis focuses on strategies within the US, utilizing the country’s large-scale whole blood and plasma collection infrastructure, which we collectively refer to as the “US blood supply.” This system engages millions of donors across diverse geographic and demographic backgrounds, potentially enabling greatly improved detection of novel pathogens. Our analysis does not address sampling strategies outside of the US blood supply, like lab discards from diagnostic testing or research specimens from blood biobanks[1].
We conducted a literature review to evaluate potential sampling approaches within the US blood supply, assessing them based on biosurveillance heuristics we’ve previously written about (4) and identified elsewhere in the literature (5,6). Key considerations include the size and demographics of the catchment population, the time between sample collection and availability for downstream analysis, and the potential to access anonymized samples routinely and cost-effectively.
Our review identified several potential strategies within the whole blood and plasma collection systems. We focus on two particularly promising approaches: plasma manufacturing pools from the plasma industry and leftover whole blood mini-pools from the transfusion medicine field.
While the NAO currently prioritizes wastewater and pooled swab sampling, we may pilot blood-based sampling if it proves sufficiently promising and wet lab capacity permits. By outlining these strategies, we also hope to stimulate interest from organizations that may be well-positioned to implement pathogen-agnostic detection programs.
US Blood Supply
The United States blood supply system serves two primary purposes: providing blood products for medical transfusions and manufacturing plasma-derived medicinal products. This system consists of the transfusion medicine field and the plasma industry, both regulated by the FDA’s Center for Biologics Evaluation and Research (CBER). While these sectors share common operational stages—collection, testing, and processing—they differ in their primary collection methods, organizational structures, and end-use applications. This overview of the US blood supply system provides context for evaluating potential biosurveillance strategies within it.
Transfusion Medicine
Transfusion medicine is a specialized healthcare field focused on the therapeutic use of blood products, which are transferred intravenously to patients (7). The primary blood products used in transfusion medicine are whole blood donations or whole blood donation-derived components such as as red blood cells, plasma, and certain types of white blood cells[2](10). Clinical applications of transfusion medicine include treating acute blood loss from trauma, surgery, sickle cell disease, and autoimmune diseases (11–13).
The transfusion medicine field primarily relies on voluntary, unpaid donors to maintain a safe blood supply, with most collection organizations operating as non-profit or not-for-profit entities (14). The lack of monetary incentives is intended to help minimize the risk of donors misrepresenting information during pre-screening (15). To prevent anemia, donors must observe an eight-week interval between whole blood donations (14,16).
Whole blood donations are collected at blood drives, blood centers, and hospital blood banks (14). As of 2024, the FDA database lists 168 blood centers and 459 hospital blood banks in the US. Blood drives are typically organized in partnership with the American Red Cross or organizations like Vitalent at locations such as schools and workplaces (17).
Two major entities behind blood collection for transfusion medicine in the United States are the American Red Cross and America’s Blood Centers (ABC). The American Red Cross, a single national organization, collects approximately 40% of the blood supply. ABC, an association of independent, community-based blood centers, accounts for the remaining 60% (18,19). These community blood centers often belong to regional networks, which in turn are members of ABC.
While ABC advocates for blood centers at the government level, a separate organization, Blood Centers of America (BCA), coordinates the transportation of blood from centers to hospitals. Many blood centers hold memberships in both ABC and BCA. The Association for the Advancement of Blood & Biotherapies (AABB) is a not-for-profit association that represents individuals and institutions involved in transfusion medicine, providing standards, accreditation and education programs.
Whole blood donation in the US involves a substantial donor base. In 2021, an estimated 6.5 million people, or about 3% of eligible donors, contributed blood products[3] (18). The National Blood Collection and Utilization Survey reported approximately 9.8 million whole blood donations that same year (20,21).
Plasma Industry
The plasma industry specializes in manufacturing plasma-derived medicinal products [4](PDMPs), which are used to treat a variety of conditions including infectious diseases, clotting disorders, and inflammatory diseases (22–24).
Plasma for PDMP production comes in two forms: source plasma and recovered plasma [5]. Source plasma, collected directly via plasmapheresis, accounts for 80% of plasma used in PDMPs. The remaining 20% is recovered plasma, extracted by centrifuging whole blood donations (25,29–32)
Unlike the non-profit transfusion medicine field, the plasma industry operates on a for-profit model. Donors receive monetary compensation, typically ranging from $30 to $70 per donation, depending on location and demand (33). Monetary incentives are made possible in part by viral inactivation techniques used during manufacturing, which significantly reduce infection risks in PDMPs compared to whole blood transfusions (34,35).
Plasma donation primarily occurs at dedicated plasma centers, which exclusively collect plasma through plasmapheresis (36). As of 2024, the FDA database lists 1,225 plasmapheresis centers in the US. The industry is dominated by three private companies: Takeda (Japan), Grifols (Spain), and CSL (Australia) (37,38). These companies often maintain vertical integration, owning facilities from collection through manufacturing, in contrast to the more decentralized transfusion medicine field (24). The Plasma Protein Therapeutics Association (PPTA) serves as a trade association for the plasma industry.
The US plasma industry has a substantially larger donor base and collection volume compared to the transfusion medicine sector. In 2019, companies operating in the US collected approximately 53.5 million plasma donations, nearly five times the number of whole blood donations (39). An estimated 20 million people in the US donate plasma annually to the plasma industry, roughly triple the number of whole blood donors (40). The US also collects approximately 70% of the world’s plasma supply for PDMP (41).
Donor screening and testing procedures
Under federal law, the Center for Biologics and Research (CBER) oversees the safety of blood products in the United States[6]. This oversight extends to both the transfusion medicine field and the plasma industry.
Federal regulations require all blood product donors to meet specific health criteria and pass a medical screening. The pre-donation process includes a detailed donor history questionnaire[7] to identify and exclude high-risk individuals, followed by a basic health screening at the beginning of the donor visit, measuring vital signs such as temperature, pulse, and hemoglobin levels (42). This screening process means that the blood supply selects for healthy or asymptomatic donors, likely negatively impacting the detection of certain pathogens.
Post-collection, donations undergo pathogen testing tailored to their intended use (43). CBER maintains a list of Transfusion-Transmitted Infections (TTIs) in the Code of Federal Regulations (CFR), defining pathogens that are both dangerous and transmissible through whole blood. Both whole blood and plasma donations are tested for TTIs, with the extent of testing depending on the product’s end use (14,44,45).
Whole blood intended for transfusion undergoes more extensive pathogen testing than plasma intended for the plasma industry, primarily due to the lack of viral inactivation in transfusion products. However, both whole blood and plasma samples are universally screened for several human-infecting viruses, including human immunodeficiency virus type 1 (HIV-1) (46), hepatitis B virus (HBV) (47), and hepatitis C virus (HCV) (48).
CBER continually adapts blood safety protocols by approving and mandating new pathogen tests in response to emerging threats. All pathogen detection assays must receive CBER approval[8]. Current assays target specific pathogens, detecting either viral nucleic acid or antibody signatures. CBER may also temporarily enforce screening and testing for emerging outbreaks, such as SARS-CoV-2 or Zika virus, until the pathogen is deemed no longer a significant threat to the blood supply (49,50).
Sample pooling is often employed to increase the efficiency of plasma testing. Multiple samples are combined into a single pool for initial screening, with individual testing performed only if the pool tests positive (43). Donors testing positive may face temporary or permanent exclusion from donation, depending on the pathogen. Their donations are not used in any blood products and may be discarded (45).
Evaluating sampling strategies
We performed a literature review of blood-based sampling strategies within the US blood supply system for biosurveillance purposes to evaluate promising sampling approaches. We prioritized approaches that offered broad donor coverage with many people contributing, sampled before pathogen inactivation, and would be feasible to access without partner organizations incurring significant extra cost. This investigation revealed several potential strategies: sampling from individual whole blood testing aliquots, source plasma testing aliquots, whole blood ‘mini-pools,’ and plasma manufacturing pools. We examine these approaches below.
Whole blood approaches
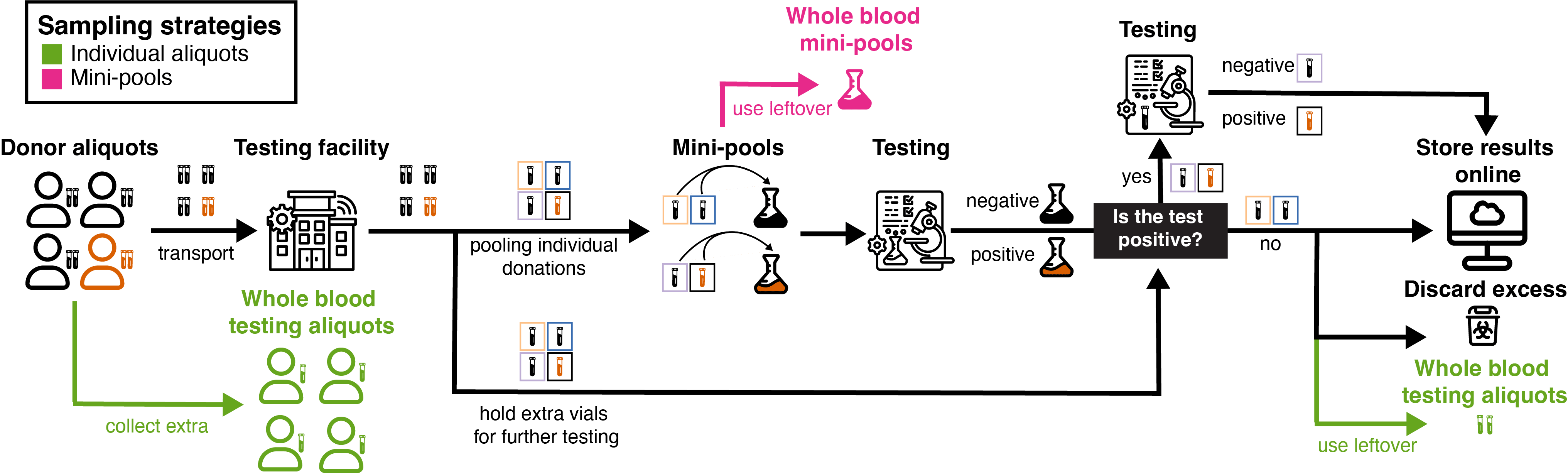
Figure 1: Whole blood donation within the transfusion medicine field.
The whole blood donation process offers two promising sample types for biosurveillance: leftover individual aliquots and mini-pools. These approaches leverage existing infrastructure and processes in the blood collection system, potentially enabling rapid and efficient pathogen detection. With approximately 6.5 million annual donors in the United States, this approach provides broad population coverage for biosurveillance efforts.
During whole blood donation, 2-4 additional aliquots (2-5 mL each) are drawn alongside the main donation for Transfusion-Transmitted Infection (TTI) testing [9](14,52,53). The samples are maintained at 1-10°C until analysis (54). To optimize efficiency, the testing system utilizes “mini-pools” comprising 4-24 individual samples (43,55). This approach allows for rapid initial screening, with positive mini-pools triggering subsequent analysis of individual samples to pinpoint the source. Both individual aliquots and mini pools could be repurposed for biosurveillance after completing their primary screening role.
Testing procedures vary based on the collection site. Hospital blood banks and blood centers typically conduct on-site testing, while samples from blood drives are sent to centralized facilities. The American Red Cross reports a 24-hour turnaround time for test results (56), indicating that samples could be quickly accessible for biosurveillance purposes. Organizations such as the Red Cross, community hospitals, and blood centers are well-positioned to facilitate access to these samples for metagenomic sequencing. Certain facilities like hospitals may even have the capacity to perform the sequencing in-house.
Plasma approaches

Figure 2: Source plasma donation within the plasma industry.
The source plasma donation process offers two potential sampling strategies for biosurveillance: individual plasma aliquots and manufacturing pools. The plasma industry engages approximately 20 million annual donors in the United States, providing an even larger donor base than whole blood donations and offering extensive population coverage for biosurveillance efforts.
After a source plasma donation, small aliquot(s) of plasma are taken directly from the main donation and used for TTI testing[10]. The plasma aliquots are cooled below 10 °C (31) and sent to a testing facility, where individual donations are tested for TTIs. At the same time, the main donation is frozen at -20 °C and sent to a storage facility where it remains for 60 days[11] before further processing (31). CBER enforces this waiting period to allow for additional information to come back which may disqualify the donation, such as symptoms of illness or a positive test for TTIs.
After the 60-day waiting period, donations are brought to the manufacturing facilities where they are combined and processed. Individual samples are thawed and pooled, into large “manufacturing pools”, ranging from 2,000 to 60,000 donors (25,58). The plasma is then processed, concentrating plasma proteins and conducting viral inactivation via dry-heat, solvent-detergent, and nanofiltration methods (59).
Manufacturing pools offer the advantage of streamlining access to many donors in a single sample, reducing sample processing requirements. However, the 60-day delay before pooling poses a significant challenge for biosurveillance, as it could substantially impede the early detection of rapidly spreading pathogens (60). Individual plasma aliquots for TTI testing are likely available earlier and may offer a faster route for biosurveillance, albeit with smaller sample sizes.
Access to source plasma samples would need to be facilitated by private companies, unlike whole blood donations which are primarily managed by non-profit organizations. This difference in organizational structure may influence the ease of implementing biosurveillance strategies within the plasma industry.
Conclusion
This article explored the US blood supply system’s potential for enabling large-scale, pathogen-agnostic biosurveillance. We examined the transfusion medicine field and plasma industry, evaluating sample types based on donor population size, pooling potential, and accessibility. Our analysis identified four promising strategies: individual whole blood testing aliquots, source plasma testing aliquots, whole blood ‘mini-pools,’ and plasma manufacturing pools.
Among these approaches, partnering with large non-profit organizations like the American Red Cross or regional blood centers for access to whole blood mini-pools or individual testing aliquots appears most promising. These samples are available shortly after donation and, being whole blood, may enable detection of intracellular pathogens.
In a future post, we plan to re-analyze metagenomic sequencing data from various blood-based sample types. This analysis will help identify detectable pathogens in blood and estimate the sequencing depth required for novel pathogen detection.
References
1. Slavov SN. Viral Metagenomics for Identification of Emerging Viruses in Transfusion Medicine. Viruses. 2022 Nov;14(11):2448.
2. Cebriá-Mendoza M, Bracho MA, Arbona C, Larrea L, Díaz W, Sanjuán R, et al. Exploring the Diversity of the Human Blood Virome. Viruses [Internet]. 2021 Nov 21;13(11). Available from: http://dx.doi.org/10.3390/v13112322
3. Stremlau MH, Andersen KG, Folarin OA, Grove JN, Odia I, Ehiane PE, et al. Discovery of novel rhabdoviruses in the blood of healthy individuals from West Africa. PLoS Negl Trop Dis. 2015 Mar 17;9(3):e0003631.
4. Bradshaw W, Grimm S. Comparing sampling strategies for early detection of stealth biothreats [Internet]. 2023 [cited 2024 Jun 7]. Available from: https://naobservatory.org/reports/comparing-sampling-strategies-for-early-detection-of-stealth-biothreats
5. Ko KKK, Chng KR, Nagarajan N. Metagenomics-enabled microbial surveillance. Nat Microbiol. 2022 Apr;7(4):486–96.
6. Morton L, Creppage K, Rahman N, Early J, Hartman L, Hydrick A, et al. Challenges and Opportunities in Pathogen Agnostic Sequencing for Public Health Surveillance: Lessons Learned From the Global Emerging Infections Surveillance Program. Health Security [Internet]. 2023 Dec 6; Available from: https://www.liebertpub.com/doi/full/10.1089/hs.2023.0068
7. Marcucci C, Madjdpour C, Spahn DR. Allogeneic blood transfusions: benefit, risks and clinical indications in countries with a low or high human development index. Br Med Bull. 2004 Jan 1;70(1):15–28.
8. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020 Jun 2;130(6):2757–65.
9. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020 Apr 1;20(4):398–400.
10. Basu D, Kulkarni R. Overview of blood components and their preparation. Indian J Anaesth. 2014;58(5):529–37.
11. Cap AP, Beckett A, Benov A, Borgman M, Chen J, Corley JB, et al. Whole Blood Transfusion. Mil Med. 2018 Sep 1;183(suppl_2):44–51.
12. Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet Transfusion: A Clinical Practice Guideline From the AABB. Ann Intern Med. 2015 Feb 3;162(3):205–13.
13. Zanatta E, Cozzi M, Marson P, Cozzi F. The role of plasma exchange in the management of autoimmune disorders. Br J Haematol. 2019 Jul;186(2):207–19.
14. McCullough JJ, editor. Transfusion medicine. Fifth edition. Hoboken, NJ Chichester: Wiley Blackwell; 2021. 580 p.
15. Voluntary blood donation: foundation of a safe and sufficient blood supply. In: Towards 100% Voluntary Blood Donation: A Global Framework for Action. World Health Organization; 2010.
16. Baart AM, van den Hurk K, de Kort WLAM. Minimum donation intervals should be reconsidered to decrease low hemoglobin deferral in whole blood donors: an observational study. Transfusion . 2015;55(11):2641–4.
17. Why Host a Blood Drive [Internet]. [cited 2024 Aug 27]. Available from: https://www.redcrossblood.org/hosting-a-blood-drive/learn-about-hosting/why-host-a-blood-drive.html
18. American Blood Centers. U.S. Blood Donation Statistics and Public Messaging Guide. 2024.
19. America’s Blood Centers. About. 2019 Feb 21 [cited 2024 Aug 2]; Available from: https://americasblood.org/about/
20. Free RJ, Sapiano MRP, Chavez Ortiz JL, Stewart P, Berger J, Basavaraju SV. Continued stabilization of blood collections and transfusions in the United States: Findings from the 2021 National Blood Collection and Utilization Survey. Transfusion . 2023;63(S4):S8–18.
21. Kracalik I, Sapiano MRP, Wild RC, Chavez Ortiz J, Stewart P, Berger JJ, et al. Supplemental findings of the 2021 National Blood Collection and Utilization Survey. Transfusion . 2023;63(S4):S19–42.
22. Benjamin RJ, McLaughlin LS. Plasma components: properties, differences, and uses. Transfusion . 2012;52(s1):9S – 19S.
23. Strengers PFW. Challenges for Plasma-Derived Medicinal Products. Transfus Med Hemother. 2023 Apr 12;50(2):116–22.
24. Marketing Research Bureau [Internet]. 2021 [cited 2024 Aug 23]. The Plasma Industry. Available from: https://marketingresearchbureau.com/the-plasma-industry/
25. Burnouf T. Modern Plasma Fractionation. Transfus Med Rev. 2007 Apr 1;21(2):101–17.
26. Hartmann J, Klein HG. Supply and demand for plasma-derived medicinal products - A critical reassessment amid the COVID-19 pandemic. Transfusion. 2020 Nov;60(11):2748–52.
27. 21 CFR Part 640 Subpart D -- Plasma [Internet]. [cited 2024 Aug 27]. Available from: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-F/part-640/subpart-D
28. U.S. Food and Drug Administration. 21 CFR Part 640 Subpart G -- Source Plasma [Internet]. 1985. Available from: https://www.ecfr.gov/current/title-21/part-640/subpart-G
29. Rivera J, Lozano M. Plasmapheresis and Plasma Donation: Challenges in the Blood/Plasma Supply Chain. Plasmatology. 2022 Jan 1;16:26348535221107565.
30. Burnouf T, Seghatchian J. “Go no Go” in plasma fractionation in the world’s emerging economies: Still a question asked 70 years after the COHN process was developed! Transfus Apher Sci. 2014 Oct;51(2):113–9.
31. 21 CFR Part 640 Subpart G -- Source Plasma [Internet]. [cited 2024 Aug 26]. Available from: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-F/part-640/subpart-G
32. Schmidt AE, MacKercher J, Youngling B, Simon TL. Source plasma deferral trends: A 3‐year analysis of 255 centers in the United States. J Clin Apher. 2022 Feb;37(1):31–9.
33. Gallagher J, Emily G. Blood Money: The Financial Implications of Plasma Sales for Individuals and Non-Bank Lenders [Internet]. Washington University in St. Louis; 2022. Available from: https://www.fdic.gov/analysis/cfr/consumer/2022/papers/gallagher-paper.pdf
34. Burnouf T. Current status and new developments in the production of plasma derivatives. ISBT Sci Ser. 2016;11(S2):18–25.
35. Hosseini KM, Ghasemzadeh M. Implementation of Plasma Fractionation in Biological Medicines Production. Iran J Biotechnol. 2016 Dec;14(4):213.
36. Rosa-Bray M, Bounpheng MA, Wisdom C, Gierman T, Becker M, Crookston KP. A Retrospective Study Establishing Pre-Pandemic Demographic Baselines for United States Source Plasma Donors. Plasmatology. 2022 Jan 1;16:26348535221129221.
37. Fortune Business Insight. Plasma Fractionation Market Size [Internet]. 2023. Available from: https://www.fortunebusinessinsights.com/industry-reports/plasma-fractionation-market-101614
38. Reeves R. Investing City. 2024 [cited 2024 Aug 23]. ADMA Biologics. Available from: https://www.business-breakdowns.com/p/adma-biologics
39. Ochoa A. The Interlinkage between Blood Plasma Donation and PThe Interlinkage between Blood Plasma Donation and Poverty in overty in the United States the United States. The Journal of Sociology & Social Welfare [Internet]. 2021 Jun; Available from: https://sites.fordschool.umich.edu/poverty2021/files/2022/07/Blood-Plasma-and-Poverty.pdf
40. McLaughlin K. Blood money. New York, NY: Simon & Schuster; 2024.
41. Hotchko M, editor. Current market landscape for plasma and immunoglobulins [Internet]. 2022. Available from: https://ipfa.nl/wp-content/uploads/2021/11/Hotchko-MRB-IPFA-EBA-Mar-2022-Redacted.pdf
42. 21 CFR 630.10 -- General donor eligibility requirements [Internet]. [cited 2024 Aug 29]. Available from: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-F/part-630/subpart-B/section-630.10
43. Dean CL, Wade J, Roback JD. Transfusion-Transmitted Infections: an Update on Product Screening, Diagnostic Techniques, and the Path Ahead. J Clin Microbiol. 2018 Jun 25;56(7):e00352–18.
44. Centers for Disease Control and Prevention. Blood Safety Basics | CDC [Internet]. 2023. Available from: https://www.cdc.gov/bloodsafety/basics.html
45. U.S. Food and Drug Administration. 21 CFR Part 610 Subpart E -- Testing Requirements for Relevant Transfusion-Transmitted Infections [Internet]. 2015. Available from: https://www.ecfr.gov/current/title-21/part-610/subpart-E
46. Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014 Jul 19;384(9939):258–71.
47. Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 2020 Mar 18;33(2):e00046–19.
48. Morozov VA, Lagaye S. Hepatitis C virus: Morphogenesis, infection and therapy. World J Hepatol. 2018 Feb 27;10(2):186–212.
49. Center for Biologics Evaluation, Research. Updated Information for Blood Establishments Regarding the COVID-19 Pandemic and Blood Donation. 2022 Aug 9 [cited 2024 Aug 2]; Available from: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/updated-information-blood-establishments-regarding-covid-19-pandemic-and-blood-donation
50. Office of the Commissioner. U.S. Food and Drug Administration. FDA; 2024 [cited 2024 Aug 2]. Zika Virus Response Updates from FDA. Available from: https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/zika-virus-response-updates-fda
51. Office for Human Research Protections (OHRP). HHS.gov. 2010 [cited 2024 Sep 4]. OHRP Expedited Review Categories (1998). Available from: https://www.hhs.gov/ohrp/regulations-and-policy/guidance/categories-of-research-expedited-review-procedure-1998/index.html
52. Blood Drawings for Human Subject Research [Internet]. 2020 [cited 2024 Aug 7]. Available from: https://www.hrpo.pitt.edu/blood-drawings-human-subject-research
53. How Much Blood is Drawn for Biochemical Blood Tests? [Internet]. [cited 2024 Aug 7]. Available from: https://en.seamaty.com/index.php?s=/sys/356.html
54. 21 CFR Part 640 -- Additional Standards for Human Blood and Blood Products [Internet]. [cited 2024 Aug 26]. Available from: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-F/part-640#p-640.2(c)(2)
55. Justiz Vaillant AA, Zubair M, Sticco KL. Transfusion Transmitted Disease. In: StatPearls [Internet]. StatPearls Publishing; 2024.
56. American Red Cross. What happens to donated blood [Internet]. 2024 [cited 2024 Aug 22]. Available from: https://www.redcrossblood.org/donate-blood/blood-donation-process/what-happens-to-donated-blood.html
57. U.S. Food and Drug Administration. Compliance Policy Regarding Blood and Blood Component Donation Suitability, Donor Eligibility and Source Plasma Quarantine Hold Requirements; Guidance for Industry. 2023.
58. Farrugia A. The Evolution of the Safety of Plasma Products from Pathogen Transmission—A Continuing Narrative. Pathogens. 2023 Feb;12(2):318.
59. Burnouf T. An overview of plasma fractionation. Annals of Blood [Internet]. 2018 Jun 1;3(1). Available from: https://aob.amegroups.org/article/view/4496
60. Hao R, Liu Y, Shen W, Zhao R, Jiang B, Song H, et al. Surveillance of emerging infectious diseases for biosecurity. Sci China Life Sci. 2022 Aug;65(8):1504–16.
- ^
Alternative blood sampling strategies include: (a) accessing anonymized lab discards from hospitals, clinics, or diagnostic companies like Quest Diagnostics and Labcorp; (b) utilizing blood biobanks such as Stemcell Technologies and PrecisionMed; and (c) direct volunteer sampling, either through at-home collection devices (e.g., those developed by Tasso) or on-site blood prick collection, similar to our current approach with nasal swabs.
- ^
Plasma, collected directly from donors via plasmapheresis, can also be used in transfusion medicine. A notable recent application was convalescent plasma therapy during the COVID-19 pandemic, where recently recovered individuals donated antibody-rich plasma to treat current patients (8,9).
- ^
Here, “blood products” includes plasma intended for transfusion medicine, but not plasma collected by the plasma industry for plasma-derived medicinal products (PDMPs).
- ^
Also known as, plasma derivatives, fractionated plasma, and plasma for fractionation.
- ^
The term ‘recovered plasma’ is used in scientific literature (14,25,26). However, the FDA uses the broader term of ‘plasma’ to encompass both recovered plasma and source plasma not intended for manufacturing (27). The FDA uses the term ‘source plasma’ to strictly refer to plasma collected through plasmapheresis intended for manufacturing (28).
- ^
The relevant section of the Federal Code of Regulations is Title 21, Chapter I, Subchapter F.
- ^
- ^
All FDA-approved tests may be found here.
- ^
According to staff from the American Red Cross of Massachusetts, the max amount of whole blood that is collected for testing is 16 mL unless otherwise noted. However, the maximum amount of blood that may be taken from a donor for testing is 50 mL (51).
- ^
The private nature of the plasma industry makes it difficult to verify how many plasma aliquots are taken, whether they are pooled, and how long after the original donation testing is completed. To verify this information, we called different locations of CSL. According to staff from CSL at the Woonsocket, Rhode Island location, one 3 mL aliquot is taken from the original plasma donation for TTI testing.
- ^
This has been reduced to 45 days during blood shortages such as those caused by COVID-19 (28,57).