Two years of doing advocacy on antimicrobial resistance
By Aanika Dalal, Mo Putera, Adnaan, DavidMcK @ 2025-11-17T22:59 (+47)
It’s been just over two years since we launched ARMoR through the CE/AIM incubation program. Last year for marginal funding week, we wrote this post to
- explain the importance of antimicrobial resistance and the need for policies that incentivise the development of novel antibiotics;
- share some of the progress we made and lessons learned; and
- make the case that our work was worth supporting.
This year, we will provide a short update to that post. In particular, we'll outline some important strategic shifts, our recent progress, and lessons learned. We’ll also summarize our current funding situation and explain what different levels of marginal funding would enable us to do.
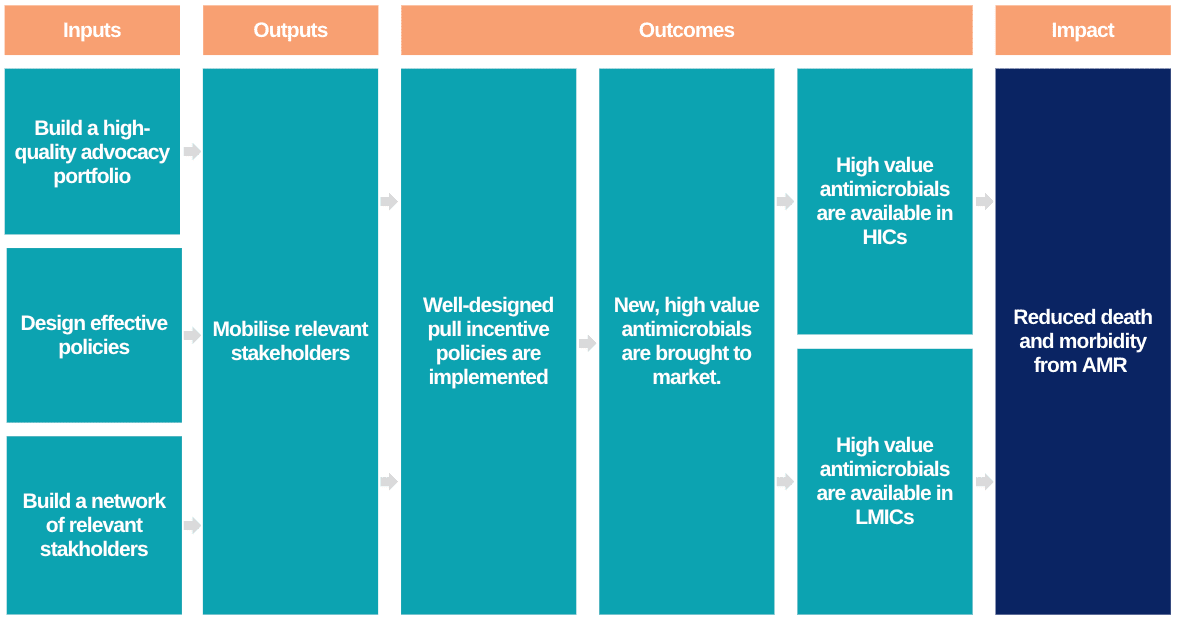
What we do; a short recap
If you are unfamiliar with what we do, I would recommend reading our post from last year's marginal funding week, but here is a quick refresher:
- Antimicrobial resistance is a serious global health threat.
- We need a continuous pipeline of new antimicrobials coming to market to replace those which are no longer effective.
- Market incentives have failed to stimulate the needed innovation.
- Significant analysis has suggested that ‘pull incentives’ could be a highly effective mechanism for reinvigorating antimicrobial R&D.
- ARMoR works to implement these policies through a combination of research, advocacy, and informational lobbying.
- We conservatively estimate that over a 10-year time horizon, our work has a cost-effectiveness of about $1,800 per life saved globally and $6,700 per life saved when just considering the EU, improving further over longer horizons as more new antimicrobials enter the market and are made accessible to patients who need them (model here)
Strategic objectives
Right now, our immediate priority continues to be getting a sufficient and well-designed pull incentive with strong global access conditions implemented in the EU. To accomplish this, we have been working at both the EU and member state level, targeting specific policy vehicles such as the EU General Pharmaceutical Legislation, EU Biotech Act, the Critical Medicines Act, Multi-Year Financial Framework, and updates to national level policies (e.g., AMR national action plans).
We decided to focus on the EU because it represents one of the largest markets for antibiotics and offers several strong policy windows to exploit. However, if high leverage opportunities arise, we will also often support organisations working on similar policies in other countries or other antimicrobial R&D ecosystem strengthening initiatives. For instance, we’ve provided analysis for the Australian Antimicrobial Resistance Network (AAMRNet) and the Swiss Roundtable on Antibiotics. Their advocacy efforts—supported in part by our work—have already driven important progress, including concrete commitments toward national pull incentive schemes.
Beyond our work in the European Union, one of the biggest strategic shifts we have made this year has been our engagement in consulting work for mission-aligned organisations. We’ve found that we can use these engagements to both deliver valuable outputs and shape the priorities of more influential actors in the AMR ecosystem. In the past year, we’ve been commissioned by organisations like the World Bank, Novo Nordisk Foundation, Union for International Cancer Control, and SECURE (a WHO initiative).
An important strategic shift we plan to make in 2026 comes from two important observations we have made about the nature of policy work:
- Policy change is stochastic. It’s difficult to back-chain. Instead, we must take robust actions that expand our ‘surface area of luck’ and be prepared for when good opportunities arise.
- Policy “capital” is transferable. Once you’ve built strong networks, a trusted brand, and deep technical expertise, you can deploy that capital across many related policy areas.
So, because policy capital transfers across adjacent domains, it often makes sense to place more than one bet in roughly the same space. For less than a doubling of cost, you can often more than double the expected impact. Over the past two years, we’ve built substantial policy capital and are now seen as credible operators in this space. In the coming year, we plan to identify and explore additional high-impact policies where that capital can be leveraged. [1]
Most notably, we received a grant from the Founder’s Pledge Catalytic Impact Fund to investigate cost-effective interventions that would increase access to both existing and novel antibiotics in LMICs. This funding allows us to explore opportunities beyond our core EU-focused pull incentive work—while still staying within the same antimicrobial R&D and access ecosystem where our existing expertise, relationships, and credibility carry over.
With that context, our priorities moving into 2026 are:
- Make significant progress toward the implementation of a well-designed pull incentive and the inclusion of strong global access conditionalities through the updates to the EU Pharmaceutical Legislation and other alternative policy vehicles.
- Engage in opportunistic, “bottom-up” efforts to influence the position of individual member states and increase support for policies and programs which support antimicrobial R&D.
- Identify and integrate cost-effective, evidence-based interventions that improve access to essential antimicrobials in LMICs as we expand our organisation.
- Build a reputable consultancy arm that can be leveraged to both produce valuable outputs and shape the priorities of more influential actors in the AMR space.
Our progress
Some wins from our first year included:
- Influenced the European Parliament’s stance on antimicrobial pull incentives, successfully incorporating language on subscription models and global access into the Parliament's position.
- Presented on incentives at a European Commission training session on antimicrobial incentives for member states and gave the keynote presentation at an event on antimicrobial incentives hosted by the UK for Nordic and Baltic countries.
- Our cost-benefit analysis on EU pull incentives has been used in discussions with 10+ governments and presented to the G7 health and finance ministers.
This year:
- We have done significant work to shape antimicrobial incentive policies in the EU, with several of our proposals now under technical discussion in the trilogue negotiations on the EU General Pharmaceutical Legislation. We currently view it as over 50% likely that our key suggestion of the adoption of an EU “subscription model” for antibiotics will be included in the final text.
- Next month, in collaboration with the UK Mission to Brussels, we will run an event on incentives for antimicrobials at the EU Parliament
- We’ve put together a EU AMR R&D Policy Coordination Group with many of the most important actors in the antimicrobial R&D ecosystem to coordinate efforts.
- Our papers are being used as sources of debate within the EU, including directly being invited to discuss with the EU Health Directorate (DG SANTE) and its economists.
- We have been commissioned by the World Bank to produce major reports and recommendations, which is now being taken to senior leadership for discussion, with strong internal support for our continued engagement. If adopted, this *could* establish World Bank financing mechanisms for global antibiotic access.
- We have been commissioned by the Novo Nordisk Foundation to produce research for the Danish Presidency of the European Council, with the goal of addressing key bottlenecks in the current policy discussions.
- We have built a strong reputation in the antimicrobial incentives space which has led to many of the key actors asking for our support and input on aligned projects. Some evidence:
- Invitations to closed-door meetings
- Easily able to get input from pretty much all the major actors in the AMR space
- Direct feedback from policymakers and other important stakeholders
- References to our work (IFPMA, EFPIA, CARB-X, etc.)
- We have been commissioned by GARDP to investigate LMIC markets and developing up new approaches to commercialisation to support antibiotic access
The impact of marginal funds
Our total budget for 2026 is $280,000. We have already secured ~50% of this budget and are looking to raise the additional $140k.
Here are some examples of what different amounts of marginal funding contributions towards this target would allow us to accomplish:
- $12,000 → funds an investigation into additional, impactful market-shaping policies we could leverage our policy capital to advocate for.
- $17,000→ funds an extra month of full-fledged operations
- $36,000 → funds a full-time senior research hire for one year
- So far, our research outputs have been responsible for some of our most promising leads and opportunities
- $90,000 → funds our entire EU advocacy campaign for 2026
Overall, we continue to believe that ARMoR is a good bet if you want to cost-effectively save lives and reduce the growing burden of antimicrobial resistance.
Please reach to aanika.dalal@armoramr.org if you have any questions, comments, feedback, or are interested in supporting our work! Thank you for your time and consideration!
- ^
If you have suggestions for high-impact biosecurity or global health R&D policies we should consider (particularly in Europe), get in touch at aanika.dalal@armoramr.org
Joey Bream @ 2025-11-18T14:12 (+5)
"$950 per life saved globally and $6,200 per life saved when just considering the EU." Incredible numbers!
OscarD🔸 @ 2025-11-27T20:28 (+2)
Nice work! I'm confused as to how 36k funds 1 senior researcher FTE year; this seems very low, what am I missing?
Mo Putera @ 2025-11-28T12:30 (+5)
CE/AIM-incubated orgs run lean. (Some past discussion here if you're interested.) I also don't live in a high CoL country, which helps.
SummaryBot @ 2025-11-18T20:01 (+2)
Executive summary: ARMoR reports two years of progress advocating for policies that incentivize new antimicrobial development, with growing influence in the EU and beyond, a stronger consultancy arm, and plans to expand into antibiotic access in low- and middle-income countries (LMICs).
Key points:
- ARMoR aims to reduce deaths from antimicrobial resistance (AMR) by promoting “pull incentive” policies that reward the development and global access of new antibiotics.
- The group focuses on shaping EU legislation and national AMR plans, currently seeing over 50% odds that an EU antibiotic subscription model will be adopted.
- ARMoR’s analysis has informed EU and G7 policy discussions and been cited by major stakeholders including IFPMA, EFPIA, and CARB-X.
- Strategic shifts include using consultancy work for groups like the World Bank and Novo Nordisk Foundation to influence priorities and expand reach.
- A new grant from the Founder’s Pledge Catalytic Impact Fund enables research on improving antibiotic access in LMICs.
- The organization seeks $140,000 in new funding for 2026, with specified marginal funding options from $12,000 to $90,000 supporting policy research, operations, or EU advocacy.
This comment was auto-generated by the EA Forum Team. Feel free to point out issues with this summary by replying to the comment, and contact us if you have feedback.