Metagenomic sequencing to screen for pathogens entering New Zealand
By Ciaran @ 2025-04-14T13:14 (+25)
Metagenomic sequencing to screen for pathogens entering New Zealand
By Ciaran O’Connell
Note: For definitions and explanations of specialised terms used in this report, please refer to the Key Terms section located at the bottom of the document, just before the references.
Executive Summary:
“The spread of pandemic viruses and invasive species can be catastrophic for human societies and natural ecosystems. SARS-CoV-2 demonstrated that the speed of our response is critical, as each day of delay permitted exponential growth and dispersion of the virus.” (A Global Nucleic Acid Observatory for Biodefense and Planetary Health, NAO Consortium, 2021).
A global Nucleic Acid Observatory was proposed in the aforementioned paper as a response to this. This project looks at the cost of implementation and other considerations within New Zealand.
“The case study dramatising the importance of early detection used a much older technology: in 2013, Israel’s poliovirus-specific environmental monitoring program detected a nascent outbreak in wastewater samples from the town of Rahat using plaque assays and swiftly initiated mass oral vaccination, eliminating the virus before even a single child came down with paralytic symptoms (Brouwer et al., 2018). Today, we have the potential to detect any virus or invasive species, even novel pandemic-class agents unknown to science, through the comprehensive metagenomic sequencing of waterways. Here we propose to build a Nucleic Acid Observatory to continuously monitor the global environment for past, present, and future pandemic viruses and invasive pests.” (NAO Consortium, 2021).
New Zealand has been sequencing urban wastewater for COVID-19. Due to ongoing technological improvements in sequencing which includes a significant reduction in cost, metagenomic sequencing is now a viable surveillance option for pandemics. “This would monitor the relative frequency of everything biological through comprehensive metagenomic sequencing of waterways and wastewater. By searching for divergences from historical baseline frequencies at various sites, viruses or invasive organisms that are undergoing exponential growth whose nucleic acids end up in the water, even those previously unknown to science could be detected.” (A Global Nucleic Acid Observatory for Biodefense and Planetary Health, NAO Consortium, 2021).
New Zealand is well suited for this proposal as it is an island nation with only 4 international airports. I propose to set up a laboratory to analyse wastewater samples taken daily from these airports. The cost of a pilot programme at Auckland airport would be roughly NZD $3.6 million with a $1.85 million upfront cost. Even if just Auckland airport was sampled, this would be a significant increase in our surveillance efforts. It would allow earlier detection and localised efforts at containment. These two factors are crucial in minimising effects both on people’s health and disruption to daily life. “Continuously monitoring nucleic acid diversity would provide us with universal early warning, obviate subtle bioweapons, and generate a wealth of sequence data sufficient to transform ecology, microbiology, and conservation” (A Global Nucleic Acid Observatory for Biodefense and Planetary Health, NAO Consortium, 2021).
Brief Explanation of Spreadsheets:
There are two corresponding spreadsheets that details the cost estimates:
Auckland only:
The pilot programme consists of one sample sequenced per day at Auckland airport which is referred to as Option 1. Option 2 is the rough estimate of three samples per day at Auckland airport and/or Auckland urban wastewater. These two options are explored because the 1.5B flow cell can only do metagenomic sequencing for one sample per sequencing run. The 1.5B flow cell has a capacity of 1.6 billion reads and one wastewater sequence requires 1 billion reads for an adequate sensitivity according to NAO Consortium, 2021.
The slightly more expensive NovaSeq X Plus sequencer can run two 1.5B flow cells each run which allows it to sequence three samples per day (1.6 billion x2 = 3.2 billion reads).
Four airports and urban areas:
An expanded programme has been estimated where samples would be taken from sites around the country and sequenced at a central hub in Christchurch.
The first case is for one 10B flow cell which can do 10 billion reads per sequencing run and therefore 10 samples. This allows for 3 samples from Auckland, 1 sample from Christchurch, Wellington and Queenstown airports, 1 sample from Auckland, Christchurch, Wellington and Queenstown urban areas.
The second case is for two 10B flow cells as the NovaSeq X Plus sequencer can run two flow cells per run. This allows for 3 samples from Auckland, Christchurch, Wellington and Queenstown airports, 2 samples from Auckland, Christchurch, Wellington and Queenstown urban areas.
These combinations of samples are not definitively the most optimal but have been explored to provide a preliminary estimate of various sequencing scenarios. I welcome any suggestions for improvement.
A variety of reagents are available, 300 cycle reagents were chosen due to better resolution for assembling genomes or identifying diverse organisms for metagenomics.
Introduction:
This project is based on the paper: A Global Nucleic Acid Observatory (NAO) for Biodefense and Planetary Health (2022). It outlines the steps and cost estimates required to build a Nucleic Acid Observatory (either standalone or as a branch of a global network) in New Zealand.
Metagenomic sequencing can be used to screen for both known and unknown foreign pathogens entering the country. “A single nucleic acid observatory monitoring site can only detect a pathogen when the frequency of the sequence fragments in question has detectably risen in an exponential pattern, but a network of sites can detect the same pattern of sequence fragments as it first becomes visible at multiple locations.” Therefore, ideally, this would ideally be done in collaboration with other nations. By monitoring their busiest global air traffic hubs and sharing results with one another, nations can detect subtle pandemic agents that might otherwise spread to most of humanity as early as possible.”, (Esvelt, 2022 Delay, Detect, Defend: Preparing for a Future in which Thousands Can Release New Pandemics). Therefore, the more nations that are part of the network, the better. However, as of 2024, no nations have implemented this.
In 2020 alone, COVID-19 cost the New Zealand government, including both direct spending on the pandemic response and economic support measures, an estimated tens of billions of dollars. A commonly cited figure is around NZD 30-40 billion, taking into account the wage subsidy, healthcare spending, and the broader economic impact.
An early detection system, though not free, would enable targeted interventions instead of costly nationwide lockdowns. Monitoring wastewater could localise responses to outbreak areas. Metagenomic sequencing can detect a wide range of known pathogens and track unknown organisms showing exponential growth, allowing for early detection of emerging diseases.
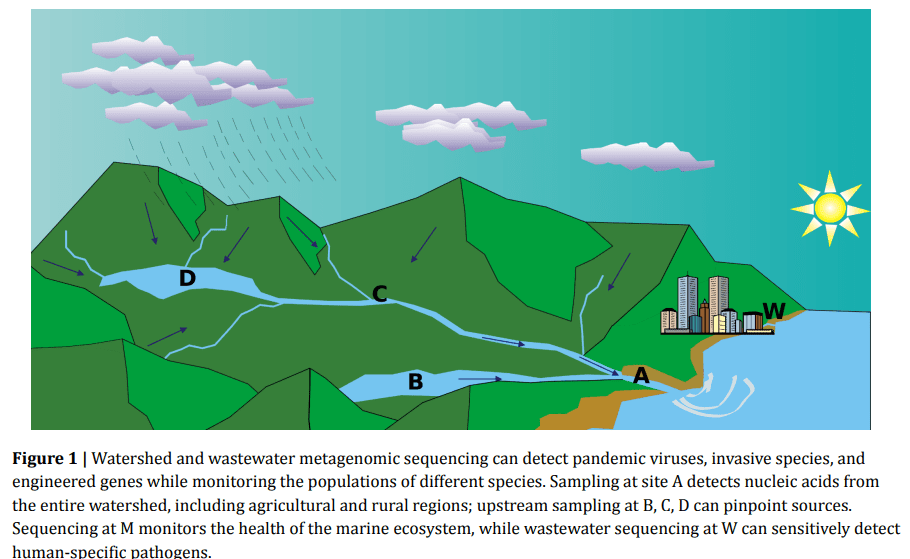
Figure 1. A Global Nucleic Acid Observatory for Biodefense and Planetary Health, NAO Consortium, 2021
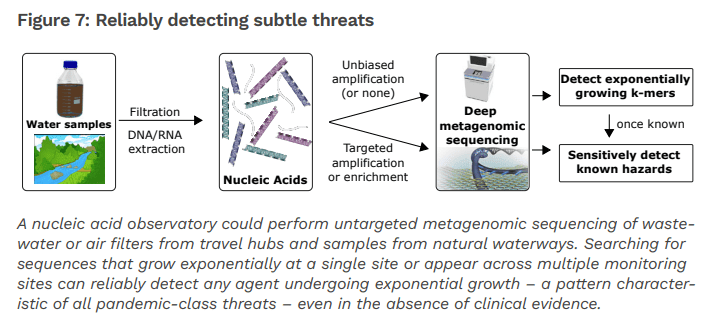
Figure 2. A Global Nucleic Acid Observatory for Biodefense and Planetary Health, NAO Consortium, 2021
Why New Zealand would be a great location to pilot this:
-Only 4 international airports (with the majority of international traffic through Auckland)
-Island country
-Low population density so outbreaks are less likely to spread to other urban areas if caught early
-High income enough to financially support such an endeavour
-Manageable number of passengers
Proposal:
I decided to focus only on international airports as they are the most likely source of pathogens due to passenger numbers. Seaports and military airports could also be sampled but these have much lower passenger numbers. Additionally, Auckland, Wellington and Christchurch have major seaports and Auckland and Christchurch airports are also used by the military. Therefore urban wastewater sampling would detect outbreaks emerging from their ports. It is worth considering if Tauranga urban wastewater should be included as it has the largest port in New Zealand.
As of 2024, New Zealand has four commercial, international airports as shown in the table below:
| City | International Passengers | Percentage of New Zealand's International Passengers | Total Passengers in 2023 |
| Auckland | 5,870,000 | 76.68% | 8,550,000 |
| Christchurch | 1,260,000 | 16.46% | 5,690,000 |
| Wellington | 290,000 | 3.79% | 710,154 |
| Queenstown | 235,000 | 3.07% | 835,196 |
| Total | 7,655,000 | 100.00% | 15,785,350 |
Note:
Running a sequencer involves a number of expensive consumables. The biggest expense is the flow cell. The smallest flow cell that could be used allows for only one sample per day (1.5B).
As shown above, Auckland airport is by far the busiest in terms of international traffic, followed by Christchurch. Wellington has international flights only to Australia and Fiji while Queenstown only to Australia.
Therefore, I propose a pilot programme of wastewater sequencing at Auckland airport daily.
This could be done initially with one sample a day (1.5B reagent kit) using an automatic composite sample. This is where a pump automatically collects a small volume of wastewater every 15 minutes over 24 hours. It is the same methodology used by ESR for COVID-19 wastewater sampling (Institute of Environmental Science and Research, n.d.)..
If the budget permits, I recommend expanding the sampling protocol to include three samples per day, utilising two 1.5B reagent kits.
This would include:
1 sample/day: Auckland airport wastewater
1 sample/day: Auckland urban wastewater
1 sample/day: Christchurch airport wastewater
The next largest flow cell is the 10B which allows for up to 10 samples per day. This would allow for expansion to Wellington and Queenstown airports as well as urban wastewater.
Various combinations can be made to balance higher temporal resolution such as taking multiple samples at the same site each day with broader geographic surveillance. Higher temporal resolution enables earlier detection of trends in pathogen numbers and provides the government with more accurate data to inform response decisions. Broader geographic surveillance allows for detection in the community (possibly indicating community transmission) and for detection at other airports, reducing the chance that a pathogen is missed. There is no ‘correct’ answer to this but personally, it seems that broader geographic surveillance should be prioritised.
Therefore with 10 samples per day, my proposal is:
3 samples/day: Auckland airport wastewater
1 sample/day: Auckland urban wastewater
1 sample/day: Christchurch airport wastewater
1 sample/day: Christchurch urban wastewater
1 sample/day: Wellington airport wastewater
1 sample/day: Wellington urban wastewater
1 sample/day: Queenstown airport wastewater
1 sample/day: Queenstown urban wastewater
This combination allows for high temporal resolution at Auckland airport as it is the most likely source of pathogens while also allowing for detection at the other international airports and the cities in which they are located for detection of community transmission spreading beyond the airport.
Further Opportunities for expansion:
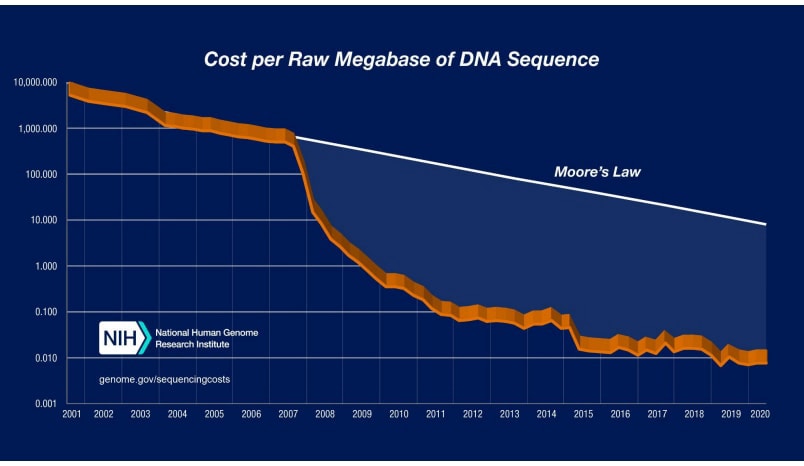
Sequencing costs for both short and long-read sequencing have dropped considerably faster than Moore's Law, a trend that looks set to continue given a variety of early-stage alternatives to current practice (Figure 3. Trends in sequencing technology. Adapted from Wetterstrand (n.d.).
Considering these trends in throughput, accuracy, and the cost of sequencing technology, further expansion of sequencing will become increasingly feasible.
Domestic Airports:
Once a successful pilot programme has been run, there is potential to expand metagenomic sequencing further. Samples could be taken from the other major domestic airports as shown in the table below.
| City | Total Passengers in 2023 | Percentage of New Zealand's Total Passengers |
| Dunedin | 920,349 | 3.67% |
| Nelson | 915,173 | 3.64% |
| Hawkes Bay | 646,096 | 2.57% |
| Palmerston North | 534,651 | 2.34% |
| Tauranga | 531,041 | 2.33% |
| New Plymouth | 401,686 | 1.61% |
| Hamilton | 304,000 | 1.00% |
This would not necessarily be done in order of passenger numbers alone as Hamilton is the fourth largest city, despite having a small airport and is in close proximity to Auckland (the most likely source of a foreign pathogen). Samples could also be taken from their corresponding urban areas resulting in coverage of all urban areas with 100,000 or more people.
Waterways:
Metagenomic sequencing can also be done on samples taken from major waterways. Human pathogens may be detected in waterways but also gene drives and invasive species.
I have not estimated the cost of sequencing the major waterways in New Zealand but it would be in the order of tens of millions of dollars at current costs. With the decrease in sequencing costs as mentioned previously and the potential for bulk purchasing discounts, waterway sequencing could become viable. The Nucleic Acid Observatory paper also discusses how sequencing could help to discover new species and to provide a snapshot of the ecological diversity at various sites. A weekly census of species abundance and genetic diversity could direct conservation resources more efficiently, and may be particularly relevant to assisting species imperilled by climate change.
In the 2022/2023 fiscal year, the New Zealand government allocated approximately NZD 110.9 million to biosecurity efforts, which include protecting agriculture from foreign pathogens. This funding was part of broader biosecurity measures designed to prevent and manage the risks posed by invasive species and diseases.
As for the impact, foreign pathogens and invasive species cause significant economic damage to New Zealand’s agriculture, with estimated costs around NZD 9.2 billion per year. This underscores the importance of robust biosecurity measures in protecting the country's primary industries. Due to the emergence of low-cost high-throughput sequencing, this should be considered alongside other biosecurity surveillance strategies.
Benefits and Risks:
The New Zealand health budget for the fiscal year 2023/2024 (1 July-30 June) was approximately NZD 29.77 billion.
In the 2023/2024 fiscal year, the New Zealand government allocated more than NZD 230 million specifically for COVID-19 and pandemic preparedness efforts. This funding is designated for a variety of activities, including:
- Vaccines and Therapeutics: Nearly NZD 200 million of this allocation is dedicated to the delivery of vaccines and the processing of PCR tests, which are crucial for ongoing COVID-19 management.
- Pandemic Surveillance: Around NZD 30 million is allocated for maintaining critical public health surveillance infrastructure, such as wastewater testing and whole-genome sequencing, to monitor and respond to potential outbreaks.
- Expansion of Pandemic Preparedness: Additional funds were set aside for initiatives like purchasing rapid antigen tests (RATs) and supporting system preparedness to identify and respond to future disease outbreaks.
These efforts are part of a broader strategy to ensure New Zealand remains equipped to handle ongoing and future health emergencies (Crimp, 2023; Kenny, 2024).
In the 2022/2023 fiscal year, Fire and Emergency New Zealand (FENZ) had a total budget of approximately NZD 668 million.
NZ spent nearly 3 times as much on fire protection than on pandemic preparedness yet COVID-19 cost the country $30-40 billion (more than the cost of the annual health budget) compared to only $618 million in fire damage in 2022. That’s about 50 times more damage caused by COVID-19. While fire damage would be significantly higher without robust fire protection measures, the same principle applies to pandemic preparedness; adequate investment in biodefense could prevent far greater losses
Why is this?
-fires are more frequent than pandemics so pandemics are easier to forget about
-fires are easier to understand in terms of prevention and spread
-only recently has technology such as metagenomic sequencing been fast, cost-effective and accurate enough to be used as a surveillance method
If New Zealand spent $3.6 million a year on the pilot programme at Auckland airport it would potentially result in the detection of pathogens at the border before community transmission is established. This could result in targeted intervention in the Auckland area, much more cost effective and politically acceptable than nationwide lockdowns as in 2020 as a response to COVID-19. Even if community transmission was established, detection days earlier allows the possibility of intervention that can dramatically reduce the spread of the virus (exponential).
What is the chance of a pandemic occurring in any given year?
| Pandemic | Estimated Deaths in NZ | Impact Classification | Notes | Sources |
| 1918 Spanish Flu | ~8,600 | Severe | One of only two severe pandemics since records began; ~2% of Māori population died | NZ History |
| 2020 COVID-19 | ~2,800 (as of early 2025) | Severe | One of only two severe pandemics since records began; $30–40B economic cost | University of Waikato |
| 2009 H1N1 (Swine Flu) | ~49 | Moderate | Mild clinical profile; ~0.62% GDP reduction | Reserve Bank of New Zealand |
| 1957 Asian Flu | Minimal | Mild | "Barely touched NZ"; low hospitalisation | Te Ara - The Encyclopedia of New Zealand |
| 1968 Hong Kong Flu | Minimal | Mild | "Barely touched NZ"; limited health impact | Te Ara - The Encyclopedia of New Zealand |
| 1890–1894 Flu Outbreak | Low hundreds annually | Low Impact | Mortality higher than usual seasonal flu; most serious pre-1918 outbreak | American Journal of Epidemiology |
| 1907 Flu Outbreak | Limited data | Low Impact | Widespread but not highly lethal; exact mortality unknown | Te Ara - The Encyclopedia of New Zealand |
Notes:
- The 1918 Spanish Flu and 2020 COVID-19 are the only severe pandemics recorded in New Zealand since consistent national records began around 1890.
- Earlier pandemics, such as rewharewha in the early 1800s, significantly affected Māori communities, but detailed quantitative data is unavailable.
- The period beginning in 1890 marks the availability of consistent national data on influenza mortality.
- Mild and low-impact pandemics have occurred more frequently. While their individual effects may seem minor, they cumulatively contribute to public health and economic burdens.
Historical Frequency: Over the past century or so, there have been several pandemics, including the 1918 Spanish flu, 1957 Asian flu, 1968 Hong Kong flu, 2009 H1N1 influenza, and the COVID-19 pandemic. This suggests that pandemics occur roughly every 10-40 years, depending on definitions and criteria used.
Annual Probability: Given this frequency, one might estimate a rough probability of 2.5% to 10% per year (1 in 40 to 1 in 10 chance). However, this is a simplistic view, and actual risk can be influenced by many factors.
Recent Estimates: The World Health Organization (2021) emphasised that pandemics are increasingly likely due to factors such as climate change, deforestation, urbanisation, and global travel. Some experts suggest that the likelihood of a significant pandemic event may now be closer to 2% to 4% annually.
I ran a Monte Carlo simulation based on historical NZ data
The Monte Carlo simulation:
- Simulated 10,000 possible 100-year futures for New Zealand.
- Each year had a chance of having no pandemic, or a low, mild, moderate, or severe pandemic.
- Many years had no pandemic at all.
- A few years had very costly events (like a severe pandemic at $35B).
The $362.8M figure is the expected cost per year, averaged across:
- All those years with no pandemic (cost = $0), and
- The rare years with massive costs (cost = $35B, $1.1B, etc.)
💡 Analogy
Think of it like insurance premiums:
- You don’t crash your car every year, but your expected cost (risk) is still baked into what you pay annually.
- Similarly, NZ doesn’t have a pandemic every year, but the risk of one occurring means we should plan as if the cost averages $362.8M/year.
So the key idea is:
- Most years = low/no cost
- Rare years = huge cost
- Average over time = $362.8M/year
| Category | Mean NZD (Per Year) | What It Represents |
| Annual Total Pandemic Cost | 362,800,000 | Average annual cost of all pandemic types based on simulation |
| Productivity Loss | 217,700,000 | Average annual cost from reduced economic activity (productivity) |
| Healthcare Cost | 145,100,000 | Average annual healthcare-related costs during pandemics |
| Mental Health Cost | 25,400,000 | Estimated mental health impact cost (7% of all pandemics) |
| Long COVID Cost | 21,600,000 | Estimated cost of long COVID from moderate/severe pandemics (6%) |
| Education Loss Cost | 14,500,000 | Estimated education and long-term income loss (4% of moderate/severe) |
| Severe Pandemic – Total Cost | 35,000,000,000 | Assumed one-time total cost of a severe pandemic (e.g. COVID-19) |
| Severe – Productivity Loss (60%) | 21,000,000,000 | Portion of severe pandemic cost attributed to productivity loss (60%) |
| Severe – Healthcare Cost (40%) | 14,000,000,000 | Portion of severe pandemic cost attributed to healthcare (40%) |
| Moderate Pandemic – Total Cost | 1,100,000,000 | Assumed one-time cost of a moderate pandemic (e.g. H1N1) |
| Moderate – Productivity Loss (60%) | 660,000,000 | Portion of moderate pandemic cost attributed to productivity loss (60%) |
| Moderate – Healthcare Cost (40%) | 440,000,000 | Portion of moderate pandemic cost attributed to healthcare (40%) |
| Mild Pandemic – Total Cost | 100,000,000 | Assumed one-time cost of a mild pandemic (e.g. 1957/1968 Flu) |
| Mild – Productivity Loss (60%) | 60,000,000 | Productivity cost share of mild pandemic (60%) |
| Mild – Healthcare Cost (40%) | 40,000,000 | Healthcare cost share of mild pandemic (40%) |
| Low Impact Pandemic – Total Cost | 50,000,000 | Assumed one-time cost of a low-impact outbreak (e.g. 1890/1907) |
| Low Impact – Productivity Loss (60%) | 30,000,000 | Productivity cost share of low-impact outbreak (60%) |
| Low Impact – Healthcare Cost (40%) | 20,000,000 | Healthcare cost share of low-impact outbreak (40%) |
1. How Much Faster Could Detection Be?
- Traditional surveillance: Relies on symptom onset, clinical testing, reporting delays (7–14+ days).
- Metagenomic sequencing: Detects known and novel pathogens 1–3 days after sample collection.
Real-world examples:
- Toronto Pearson Airport detected SARS-CoV-2 variants up to 3 weeks earlier than clinical detection.
- Wastewater sequencing in the US flagged viral spread before any cases were diagnosed in clinics.
2. How Early Detection Supports Targeted Containment With earlier alerts, health authorities can:
- Contact trace and isolate cases before community transmission
- Impose localised restrictions (e.g., on specific flights or suburbs)
- Mobilise pop-up testing and vaccine efforts
- Avoid full-scale lockdowns and border closures
3. Illustrative Impact Using COVID-19 COVID-19’s effective reproduction number (R0) was between 2.5 and 3.5, meaning cases doubled every 3–5 days during early spread.
If New Zealand had detected an outbreak 5 days earlier and acted immediately:
- Cases could have been cut in half in the first generation
- Hospital and ICU burden would have dropped proportionally
- Economic activity could have continued uninterrupted in most of the country
Modelling shows that each week of delay in lockdown cost New Zealand billions in GDP and prolonged social and health system stress.
4. Comparison of Early Intervention Effects by R0 Value
| Reproduction Number (R0) | Case Doubling Time | Cases After 10 Days (No Intervention) | Cases With 5-Day Earlier Intervention | Reduction in Cases |
| 1.5 | ~6 days | 2.8x original cases | ~1.8x original cases | ~35% fewer cases |
| 2.5 | ~3.5 days | 5.7x original cases | ~2.8x original cases | ~50% fewer cases |
| 3.5 | ~2.5 days | 10x original cases | ~3.2x original cases | ~68% fewer cases |
Faster detection flattens the curve sooner, reducing transmission, pressure on hospitals, and need for economically disruptive interventions. As we can see, the more virulent the disease, the greater the benefit of early detection via metagenomic sequencing.
Cost-Benefit Model for Pilot Programme at Auckland Airport The pilot programme will only sequence at Auckland airport, which is responsible for ~76% of New Zealand’s international traffic. If we assume (with reasonable uncertainty) that this results in a 60% detection rate of all pathogens entering through Auckland Airport, and that detection enables an early intervention that reduces the cost of a pandemic by 60–80%, the expected annual value of avoided cost is substantial.
Using the Monte Carlo-derived annual pandemic burden of $364M, the expected value of the programme is:
EV = 0.76 × 0.6 × 0.6–0.8 × $364M = $99M to $132M per year
Compared to the annual cost of just $3.6M, this implies a benefit-to-cost ratio of 27:1 to 37:1. These estimates are consistent with New Zealand Treasury data showing that a single week's delay in lockdown during COVID-19 cost nearly $875M in GDP ("A cost benefit analysis of 5 extra days at COVID-19 alert level 4").
Note: Both the detection rate and early intervention cost reduction values are my biggest uncertainty. If there is a better estimate, please suggest one.
At a cost of only $3.6 million per year, a figure that is set to decrease as sequencing becomes increasingly less expensive, the pilot programme is an absolute bargain. It is also worth noting that sequencing scales so expanding the programme would result in a lower per-sample cost.
Alternative method:
According to Foox et. al. (as cited in NAO Consortium, 2021), short-read sequencing based on Illumina technology is currently the most cost-effective on a per-read basis and consequently offers the greatest sensitivity, meaning it may be optimal for wastewater. Therefore, the cost estimates in this paper are based on Illumina sequencing. It should be noted that other sequencing companies exist, such as Oxford Nanopore which have their own advantages (as noted below) but will not be investigated further at this stage.
Once the samples are amplified, they may be shotgun sequenced by short or long read sequencing, or both (NAO Consortium, 2021). Nanopore sequencing currently allows for much longer read length and superior recognition of gene drive systems and other constructs that combine genetic elements normally never found adjacent to one another. It also offers the ability to sequence DNA containing non-standard bases such as aminoadenine (Zhou et al., 2021; Pezo et al., 2021; Sleiman et al., 2021). Nanopore sequencing can also sequence RNA directly without reverse transcription, which is useful for monitoring RNA viruses (Grädel et al., 2020; Wongsurawat et al., 2019). Recent advances enabling adaptive nanopore sequencing to better detect low abundance samples may allow it to approach the sensitivity of short-read sequencing (Martin et al., 2021).
Instead of or in addition to wastewater, sequencing of air filters from aircraft and airports could also be done, or possibly clinical samples from flight crews (Esvelt, 2022).
Key Assumptions and Considerations:
-Only one sequencing machine(potentially risky it if breaks down)
-Laboratory space could be shared with another government lab and therefore will be free
-No bulk discounts (this is the worst case scenario but I think it is likely to get some discount given the high volume of consumables being purchased)
-300 cycle reagent flow cells are needed for metagenomics due to better resolution for assembling genomes or identifying diverse organisms
-8 lanes per flow cell but can multiplex to sequence more samples per sequencing run
Conclusion:
It is not a matter of if there will be another pandemic, but when. Due to the rapid development of new sequencing technology, we have the opportunity to reform our pandemic preparedness plan. The cost of implementation is considerable but negligible compared to the cost of a pandemic.
---------------------------------------------------------------------------------------------------------------------------
Supplementary information:
Key Terms:
Metagenomic Sequencing: A cutting-edge technology that allows scientists to identify and study the genetic material of all organisms present in a sample, including viruses, bacteria, and other microbes. It provides a comprehensive snapshot of the biological content in an environment.
Baseline Frequencies: The normal or average levels of certain organisms or biological markers in the environment. By comparing current data to these baselines, scientists can detect unusual changes that might indicate a new outbreak or the spread of an invasive species.
Reads: Short sequences of DNA or RNA that are generated during the sequencing process. The number of reads refers to how many of these sequences are produced, which can indicate how thoroughly a sample has been analysed.
Sensitivity: The ability of a sequencing process or test to detect even small amounts of a pathogen or other target organism. Higher sensitivity means more accurate detection of low-abundance organisms.
Flow Cell: A key component of a sequencing machine where the actual sequencing reaction takes place. It’s one of the most expensive consumables, with different sizes allowing for different numbers of samples to be sequenced at once.
Reagents: Chemicals or substances used in a laboratory to cause a reaction or to detect the presence of other substances. In sequencing, reagents are essential for preparing samples and enabling the sequencing process.
Genome Assembly: The process of piecing together the sequence of DNA from smaller fragments generated during sequencing. This is important for understanding the genetic makeup of organisms and detecting variations that might indicate new or emerging pathogens.
Automatic Composite Sample: A method of collecting wastewater samples where small amounts are automatically collected at regular intervals (e.g., every 15 minutes) over a 24-hour period. These small samples are combined into one composite sample for analysis.
Temporal Resolution: The frequency at which data is collected or sampled over time. Higher temporal resolution means more frequent sampling, which can lead to earlier detection of changes or trends in pathogen levels.
Moore's Law: Originally a prediction in computer science that the number of transistors on a microchip would double approximately every two years, leading to an increase in computing power. In the context of sequencing, it refers to the rapid decrease in the cost of sequencing over time.
Consumables: Supplies that are used up during the sequencing process and need to be replaced regularly. In the context of sequencing, consumables include items like flow cells, reagents, and other laboratory supplies.
Throughput: In the context of sequencing, throughput refers to the amount of data that can be processed in a given period of time. Higher throughput means more samples can be sequenced, or more data can be generated from each sample, in a shorter amount of time.
Gene Drives: Genetic engineering technologies that increase the likelihood of a particular gene being passed on to the next generation, potentially spreading that gene through a population more rapidly than through natural inheritance.
Expected Value: A calculated estimate of the average outcome over time, considering the likelihood of an event and its potential impact. In the context of pandemic prevention, it helps estimate the financial benefit of preventing a pandemic.
Shotgun Sequencing: A method where DNA is randomly fragmented and sequenced to reconstruct the entire genome. It is often used in metagenomic studies to analyse complex samples with many different organisms.
Nanopore Sequencing: A type of long-read sequencing that passes DNA or RNA molecules through a tiny pore to read their sequence. It allows for the sequencing of long DNA fragments and can directly sequence RNA, making it useful for monitoring RNA viruses.
Multiplexing: A method that allows multiple samples to be sequenced together in a single run by adding unique identifiers to each sample. This increases the efficiency of sequencing and reduces costs.
Lanes: Sections of a flow cell that can be used to run multiple sequencing reactions simultaneously. By using multiple lanes, labs can sequence more samples in a single run, improving efficiency.
Airport ownership:
Possibly irrelevant but could be useful for approval of wastewater treatment plans
Auckland:
Public company
Christchurch:
Christchurch City Council (75%)
NZ Government (25%)
Wellington:
Infratil (66%)
Wellington City Council (34%)
Queenstown:
Queenstown Lakes District Council (75.01%)
Auckland International Airport Limited (24.99%)
Military Airports:
The New Zealand Defence Force (NZDF) primarily uses several key airports for international military flights. These include:
- Auckland Airport: Auckland Airport's military section, known as Whenuapai Air Base (RNZAF Base Auckland), is a major hub for the Royal New Zealand Air Force (RNZAF) and handles both domestic and international military flights.
- Ohakea Air Base: Located near Palmerston North, Ohakea Air Base is another primary RNZAF base that supports both domestic and international military operations.
- Christchurch Airport: Christchurch International Airport has a military section that is used by the RNZAF, particularly for operations related to the Antarctic missions (flights to and from Antarctica) as well as other international operations.
- Woodbourne Air Base: Near Blenheim in the South Island, Woodbourne Air Base also supports military aviation, although it primarily handles domestic military operations. However, it can be used for international flights when necessary.
In total, these four locations are the primary airports used by the New Zealand military for international flights. While other airports might occasionally be used for specific operations or exercises, these are the main bases equipped to handle regular military air traffic, including international missions. These could also be considered for sequencing, although Christchurch and Auckland airports would already be covered through the pilot programme.
Seaports:
Ranking of New Zealand's seaports by cargo volume in 2023, listed in order:
- Port of Tauranga: 25.6 million tonnes of cargo (Port of Tauranga Limited).
- Lyttelton Port (Christchurch): 15.7 million tonnes of cargo (Port of Tauranga Limited).
- Port of Auckland: 11.0 million tonnes of cargo (Port of Tauranga Limited).
- Napier Port: 5.4 million tonnes of cargo (Port of Tauranga Limited).
- Port Taranaki (New Plymouth): 4.0 million tonnes of cargo (Port of Tauranga Limited).
- Marsden Point (Northport): 3.5 million tonnes of cargo (Port of Tauranga Limited).
- Port Otago (Dunedin): 3.2 million tonnes of cargo (Port of Tauranga Limited).
- Bluff Port: 1.9 million tonnes of cargo (Port of Tauranga Limited).
- Timaru Port: 1.5 million tonnes of cargo (Port of Tauranga Limited).
- Whangarei Port: 1.2 million tonnes of cargo (Port of Tauranga Limited).
- Nelson Port: 1.0 million tonnes of cargo (Port of Tauranga Limited).
- Gisborne Port: 0.8 million tonnes of cargo (iContainers).
- Picton (Marlborough Sounds): Less than 1.0 million tonnes of cargo, mostly related to ferry services (iContainers).
References:
Consortium, T.N. (2021). A Global Nucleic Acid Observatory for Biodefense and Planetary Health.
Esvelt, K. M. (2022). Delay, detect, defend: Preparing for a future in which thousands can release new pandemics (Geneva Paper No. 29/22).
Foox, J., et al. (2020). Multi-platform assessment of DNA sequencing performance using human and bacterial reference genomes in the ABRF next-generation sequencing study. bioRxiv. https://doi.org/10.1101/2020.07.23.218602
Zhou, Y., et al. (2021). A widespread pathway for substitution of adenine by diaminopurine in phage genomes. Science, 372(6541), 512–516. https://doi.org/10.1126/science.abf1316
Pezo, V., et al. (2021). Noncanonical DNA polymerization by aminoadenine-based siphoviruses. Science, 372(6541), 520–524. https://doi.org/10.1126/science.abf1505
Sleiman, D., et al. (2021). A third purine biosynthetic pathway encoded by aminoadenine-based viral DNA genomes. Science, 372(6541), 516–520. https://doi.org/10.1126/science.abf1315
Grädel, C., et al. (2020). Whole-genome sequencing of human enteroviruses from clinical samples by nanopore direct RNA sequencing. Viruses, 12(4), 1-13. https://doi.org/10.3390/v12040351
Wongsurawat, T., et al. (2019). Rapid sequencing of multiple RNA viruses in their native form. Frontiers in Microbiology, 10, 260. https://doi.org/10.3389/fmicb.2019.00260
Institute of Environmental Science and Research. (n.d.). COVID-19 wastewater dashboard. ESR. Retrieved August 28, 2024, from https://www.esr.cri.nz/digital-library/wastewater-dashboard/
Wetterstrand, K. A. (n.d.). The cost of sequencing a human genome. National Human Genome Research Institute. Retrieved August 31, 2024, from https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost
Kenny, K. (2024, June 6). RATs, vaccines and treatments, the budget for Covid: What you need to know. RNZ. https://www.rnz.co.nz/news/what-you-need-to-know/518820/rats-vaccines-and-treatments-the-budget-for-covid-what-you-need-to-know
Crimp, L. (2023, November 22). Comprehensive pandemic framework created. RNZ. https://www.rnz.co.nz/news/national/502996/comprehensive-pandemic-framework-created
World Health Organization. (2021). Global preparedness monitoring board annual report 2021: From worlds apart to a world prepared. World Health Organization. https://www.who.int/publications/i/item/9789240034089
Sources:
- NZ History - The 1918 Influenza Pandemic
- University of Waikato - Opinion: 'Safety at all costs' - costs lives
- Reserve Bank of New Zealand - Swine flu: what are the impacts on the New Zealand economy
- Te Ara - The Encyclopedia of New Zealand - Epidemics
- American Journal of Epidemiology - Influenza in New Zealand Before 1918: A Preliminary Report
slg @ 2025-07-17T14:50 (+4)
Simon from the Nucleic Acid Observatory here. Thanks for writing this up, exciting to have more people thinking about this.
For your proposal, can you say more about:
i) how would you envision getting access to these samples? Airport wastewater is great, but getting permits can be tricky.
ii) what would a pilot project of your proposal look like? I.e., what kind of sequencing would you do, where would you do the sequencing, who would do processing?
For your NovaSeq X numbers, one thing worth noting: You can normally only buy a full 25B flow cell, not just a ~1.5B lane. The Broad offers this, but I'm unsure how common that offer is. I assume it's reate. Now, you might be fine with a smaller sequencer anyway, as airplane wastewater has higher relative abundance. But this kind of stuff is pretty important for unit economics: to fill a full Novaseq X flow cell you'd need several samples, which can be tricky, especially when just starting a new program.
Swan 🔸 @ 2025-04-17T11:25 (+4)
Thanks for writing this up! Have you considered condensing this into a two-page policy brief? I am sure this could also be useful as a template for other countries. Feel free to dm me.
Ciaran @ 2025-04-23T09:53 (+1)
Thanks Swan. I have thought about doing that although I haven't written a policy brief before. It seems like this model of screening airports would work best in island countries such as Australia, and I could probably adapt this report for that context. My greatest uncertainty is in the estimate for cost savings as this depends on predicting the frequency and severity of future pandemics. Do you have any feedback on this area?
Swan 🔸 @ 2025-04-25T19:55 (+1)
Will DM :)
Karen Singleton @ 2025-04-16T02:55 (+2)
Thank you for writing this comprehensive proposal. I agree with your conclusion it's not a case of if but when and we should be improving our pandemic planning now.
Industrial animal agriculture creates conditions where pathogens can evolve and spread rapidly between densely housed animals, potentially creating new zoonotic diseases that can jump to humans. This factor alone raises the likelihood of future pandemics and strengthens the case for robust early detection systems.
The comparison to fire protection spending provides a compelling perspective. It's striking that New Zealand spent nearly 3 times more on fire protection than pandemic preparedness, despite COVID-19 costing the country roughly 50 times more than annual fire damage. This kind of data-driven comparison makes a strong case for increasing pandemic surveillance investment.
I hope you're able to get this information to MoH!
Ciaran @ 2025-04-23T09:59 (+2)
Thank you Karen. I have been in contact with people at Health New Zealand and at ESR but unfortunately, due to the current administration's cuts to the budget they think it is unlikely to be implemented anytime soon. Feedback from one scientist at ESR was that the sequencing cost was still too high, although perhaps he would change his mind when the costs are compared to the estimated damages.
In regard to industrial animal agriculture, this may be a problem in New Zealand that I am ignorant of, but it seems most likely the pathogen would emerge internationally rather than locally, hence my focus on border detection, although you make a good point and I'm glad that area is not being completely neglected.
SummaryBot @ 2025-04-14T15:01 (+2)
Executive summary: This exploratory proposal advocates for a pilot programme using metagenomic sequencing of wastewater at Auckland Airport to detect novel pathogens entering New Zealand, arguing that early detection could avert the enormous health and economic costs of future pandemics at a relatively low annual investment of NZD 3.6 million.
Key points:
- Pilot proposal: The author proposes a metagenomic sequencing pilot focused on Auckland Airport—responsible for 77% of international arrivals—using daily wastewater sampling to detect both known and novel pathogens.
- Cost-benefit analysis: A Monte Carlo simulation suggests that the expected annual pandemic cost to New Zealand is NZD 362.8 million; even partial early detection (e.g., 60% at Auckland) could yield NZD 99–132 million in avoided costs annually, implying a benefit-cost ratio of up to 37:1.
- Technology readiness: Advances in sequencing technology (e.g., Illumina and Nanopore) have reduced costs and increased sensitivity, making real-time pathogen surveillance more feasible and scalable than ever before.
- Pandemic risk context: Based on historical data and WHO warnings, the annual probability of a severe pandemic may range from 2–4%, reinforcing the need for proactive surveillance.
- Expansion potential: The framework could later include additional international and domestic airports, urban wastewater, and even waterways, enhancing both temporal and geographic surveillance coverage.
- Policy rationale: Current pandemic preparedness spending is relatively low compared to the costs of past pandemics, and the public intuitively supports visible, understandable risks (like fire), underscoring the need to invest in less tangible but equally critical threats like pandemics.
This comment was auto-generated by the EA Forum Team. Feel free to point out issues with this summary by replying to the comment, and contact us if you have feedback.