Human and HPV Genes Combine to Form Extrachromosomal DNA that Promotes Oropharyngeal Tumor Growth
By Connor Wood @ 2025-04-18T02:09 (–2)
The Viral-Genomic Synergy Behind Cancer
Oropharyngeal cancer (OPC), a subset of head and neck cancers, has witnessed a sharp rise in incidence, largely driven by infection with high-risk strains of human papillomavirus (HPV). Once known primarily for its link to cervical cancer, HPV has now emerged as a dominant etiological factor in oropharyngeal squamous cell carcinoma (OPSCC), especially among younger and non-smoking populations.
At the heart of this oncogenic process lies an intricate mechanism involving extrachromosomal DNA (ecDNA) — circular DNA fragments that exist outside the standard human chromosomes. Recent studies have illuminated a striking biological phenomenon: human and HPV genes combine to form extrachromosomal DNA that promotes oropharyngeal tumor growth. This blog delves into the significance of this hybrid ecDNA, its role in tumorigenesis, and its potential as a target for novel therapies.
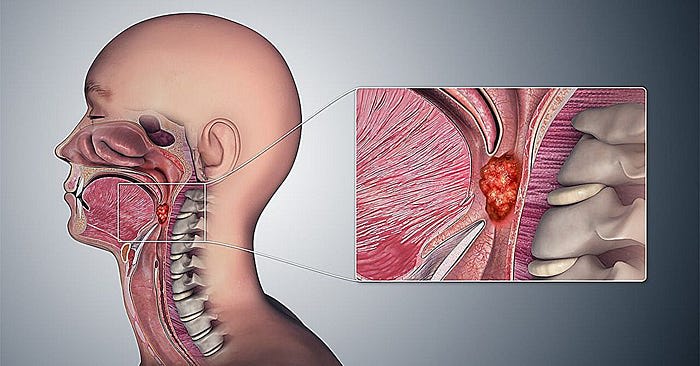
What Is Extrachromosomal DNA (ecDNA)?
Extrachromosomal DNA (ecDNA) refers to circular DNA fragments found in the nuclei of cancer cells, separate from the standard chromosomal genome. Unlike chromosomal DNA, ecDNA can replicate independently and is often enriched with oncogenes — genes that drive cancer development.
EcDNA is increasingly recognized as a key driver of tumor evolution in multiple cancer types, including glioblastoma, lung cancer, and now, HPV-positive oropharyngeal cancer. These DNA circles often contain multiple copies of oncogenes, contributing to uncontrolled cell growth and therapy resistance.
In HPV-associated cancers, ecDNA assumes a more complex role — blending viral and human genetic elements into hybrid structures that facilitate aggressive tumor behavior.
Hybrid ecDNA: A New Frontier in HPV-Associated Cancers
Prevalence in HPV-Positive OPC
Several high-throughput genomic studies have confirmed that hybrid ecDNA structures are prevalent in HPV-positive oropharyngeal cancers. These DNA circles often contain both viral oncogenes (notably E6 and E7) and sections of the human genome, including somatic enhancers — non-coding regions that regulate gene expression.
In HPV-positive tumors, these hybrid ecDNA elements act as amplified transcription hubs, dramatically increasing the expression of cancer-promoting genes. This mechanism helps explain why HPV-positive OPC, despite often arising in younger and healthier individuals, can display such aggressive clinical behavior.
Functional Impact: Oncogene Overexpression and Immune Evasion
The E6 and E7 oncogenes play pivotal roles in HPV-mediated oncogenesis. E6 degrades the tumor suppressor protein p53, while E7 inactivates retinoblastoma protein (Rb), disrupting normal cell cycle regulation.
When carried on ecDNA, these oncogenes are not only more abundantly expressed, but also less susceptible to normal cellular regulatory mechanisms. Furthermore, studies show that hybrid ecDNA can generate fusion transcripts, in which HPV promoters drive the expression of human oncogenes. This fusion creates a chimeric transcriptional landscape, allowing for:
- Enhanced cellular proliferation: The amplification of oncogenes on ecDNA leads to significantly increased cellular proliferation rates, as ecDNAs can exist in high copy numbers and exploit open chromatin structures to drive robust transcription of growth‑promoting genes. For example, ecDNA harboring amplified MYC or EGFR oncogenes has been shown to promote faster cell‑cycle progression and higher division rates compared to equivalent chromosomal amplifications, providing a clear growth advantage to tumor cells.
- Evasion of host immune responses: Hybrid ecDNA can encode not only viral oncogenes but also genes that modulate immune checkpoints or cytokine expression, reducing tumor‑associated antigen presentation and enabling tumors to avoid immune detection. Moreover, ecDNA‑driven intratumoral heterogeneity generates phenotypically diverse clones — some lacking key antigenic markers — thus allowing subsets of cells to escape host immune surveillance.
- Increased resistance to conventional therapies: EcDNA‑mediated oncogene amplification is often unevenly segregated during cell division, fostering genetic heterogeneity and the rapid emergence of drug‑resistant subclones under therapeutic pressure. In addition, the highly accessible chromatin on ecDNA permits quick reactivation of oncogene transcription following drug withdrawal, further contributing to therapy resistance and disease relapse.
Such properties make ecDNA a critical target in understanding HPV-related carcinogenesis.
Epigenetic Architecture: The Role of Enhancers
One of the defining features of hybrid ecDNA is its enrichment with enhancer elements, both viral (such as HPV’s L1 enhancer) and somatic (human-origin enhancers). These elements boost the transcriptional activity of oncogenes embedded in ecDNA, further fueling cancer progression.
Interestingly, research has revealed that the epigenetic landscape of ecDNA — its chromatin state and DNA accessibility — differs significantly from that of linear chromosomal DNA. The open chromatin structure of ecDNA makes it more transcriptionally active, contributing to the aggressive growth patterns seen in HPV-positive tumors.
Targeting these enhancer regions has been shown to reduce E6/E7 expression, indicating a potential avenue for therapy.
Clinical Implications and Therapeutic Opportunities
A New Target for Cancer Therapy
The discovery of hybrid ecDNA has opened up new therapeutic frontiers in HPV-related cancers. Traditional cancer treatments often target chromosomal mutations or protein products. However, the unique properties of ecDNA — its amplification potential, enhancer architecture, and extrachromosomal inheritance — offer distinct vulnerabilities.
Promising therapeutic strategies include:
- Disrupting ecDNA replication or maintenance using small molecules
- Targeting enhancer regions with epigenetic drugs such as BET inhibitors
- Blocking fusion transcript expression using RNA interference technologies
By interfering directly with the hybrid ecDNA, these therapies aim to suppress oncogene expression at the root, offering a potentially transformative approach for patients with ecDNA-driven cancers.
Toward Personalized Medicine
The presence of hybrid ecDNA can serve as a biomarker for stratifying patients and tailoring treatment plans. Detecting ecDNA in tumor biopsies or circulating tumor DNA (ctDNA) in blood could allow oncologists to:
- Predict tumor aggressiveness: The presence of ecDNA in tumor samples correlates strongly with more aggressive clinical features, such as higher stage at diagnosis, lymph node involvement, and reduced overall survival, making ecDNA abundance a prognostic indicator. Computational analyses have demonstrated that quantifying ecDNA levels from whole‑genome sequencing can stratify patients by risk, with higher ecDNA content predicting poorer outcomes.
- Monitor treatment response: Levels of ecDNA (or related eccDNA) detected in patient blood samples via liquid biopsy decline in response to effective therapies, mirroring reductions in tumor burden. Serial monitoring of circulating ecDNA offers a real‑time, minimally invasive method to assess treatment efficacy and guide clinical decision‑making.
- Detect early signs of recurrence: Rising ecDNA levels in circulation often precede radiographic or clinical evidence of tumor recurrence, providing an opportunity for earlier therapeutic intervention. Studies have shown that increases in circulating ecDNA can be detected weeks to months before conventional imaging modalities reveal tumor regrowth.
As precision oncology advances, incorporating ecDNA profiling could elevate clinical decision-making, particularly for HPV-associated malignancies.
Future Directions: Research and Beyond
Although the field is still evolving, ongoing research continues to uncover the structural complexity and functional diversity of hybrid ecDNA. Key areas for future exploration include:
- Mapping the full genomic architecture of ecDNA in HPV-positive cancers
- Understanding the mechanisms of ecDNA inheritance during cell division
- Developing targeted delivery systems for anti-ecDNA therapies
Moreover, studying hybrid ecDNA may provide broader insights into viral integration, epigenetic regulation, and non-chromosomal genome evolution, deepening our understanding of cancer biology as a whole.
Toward a New Paradigm in HPV-Driven Cancer Treatment
The emerging recognition that human and HPV genes combine to form extrachromosomal DNA that promotes oropharyngeal tumor growth represents a paradigm shift in our understanding of virus-associated oncogenesis. Hybrid ecDNA functions as both a structural and functional hub — amplifying oncogene expression, reshaping the epigenome, and accelerating tumor progression.
By targeting these structures, we have the potential to interrupt cancer at its genetic source, offering new hope for patients facing HPV-positive oropharyngeal cancer. As research advances, hybrid ecDNA may soon transition from a scientific curiosity to a central pillar in precision cancer therapy.
Frequently Asked Questions
Q1. What makes hybrid ecDNA different from normal chromosomal DNA?
A: Hybrid ecDNA exists outside the normal chromosome structure and typically contains both human and viral genes. It can replicate independently and is often more transcriptionally active, leading to higher expression of oncogenes involved in cancer.
Q2. How does HPV contribute to the formation of hybrid ecDNA?
A: HPV can integrate its genome into the host’s DNA, but in some cases, fragments of the viral genome instead combine with human genomic elements to form ecDNA. These hybrid circles can carry viral oncogenes like E6 and E7 alongside human enhancers, promoting aggressive cancer growth.
Q3. Can current treatments target hybrid ecDNA?
A: While current therapies don’t specifically target ecDNA, research is underway to develop drugs that disrupt ecDNA formation, maintenance, or gene expression. These treatments could offer new, more effective options for HPV-positive cancer patients in the near future.
Meet Pubmed.ai
Pubmed.ai is an innovative research tool that harnesses the power of artificial intelligence to transform how clinicians, researchers, and students access medical literature. Connected directly to the extensive PubMed database, Pubmed.ai allows users to simply enter a keyword — whether it’s a specific disease, condition, or broader medical topic — and instantly retrieve a curated list of relevant articles. The system goes beyond mere retrieval; it automatically generates a concise introduction to the topic and extracts the key takeaways from the available literature, enabling users to quickly grasp the essence of the research. This blog was written with the help of pubmed.ai, which can help scholars and researchers search for resource information more smoothly on PubMed. It can even generate a complete academic report for you. Whether you are looking to stay updated on the latest advances or conducting in-depth academic research, Pubmed.ai offers a forward-thinking solution that simplifies the navigation of vast biomedical resources. Embrace the future of medical research with Pubmed.ai, where a single keyword unlocks a world of knowledge and expertly distilled information at your fingertips.
References
- Verhaak, R.G.W., et al. (2019). “EcDNA in Cancer: Mechanisms and Clinical Implications.” Nature Reviews Cancer.
- Akagi, K., et al. (2014). “Genome-wide analysis reveals HPV integration sites and hybrid ecDNA in OPSCC.” Proceedings of the National Academy of Sciences.
- Turner, K.M., et al. (2017). “Extrachromosomal oncogene amplification drives tumor evolution and genetic heterogeneity.” Nature.
- Zheng, Y., et al. (2021). “HPV–human hybrid extrachromosomal DNA as a therapeutic target in oropharyngeal cancers.” Cancer Cell.
- Mischel, P.S., et al. (2022). “The role of circular DNA in cancer therapy resistance.” Cell Reports Medicine.
Henry Stanley 🔸 @ 2025-04-18T09:55 (+2)
Interesting stuff but I think this is a bit too technical/in the weeds for the average EA forum reader - and it's not super clear what the EA angle is here.