Pain relief: a shallow cause exploration
By Samuel Dupret, JoelMcGuire, MichaelPlant @ 2023-01-27T09:55 (+85)
This is a linkpost to https://www.happierlivesinstitute.org/report/pain-relief
This shallow investigation was commissioned by Founders Pledge.[1]
Summary
This report is a shallow cause exploration, completed in two weeks, which expands on our previous work considering pain as a potential cause area (Sharma et al., 2020)[2]. Here, we attempt to explore the relationship between pain and subjective wellbeing (SWB) more directly, both conceptually and quantitatively.
First, we try to calculate a conversion rate between self-reported pain intensity and SWB measures. However, the limited literature provides us with two potential conversion rates: a 1-point change on a 0-10 pain scale could lead to either a 0.1-point or 1-point change on a 0-10 SWB scale. Choosing one or the other leads to drastically different results when evaluating the cost-effectiveness of pain treatments.
Second, we assess the severity and scale of chronic pain in terms of life satisfaction to be large. However, we think this is likely an underestimate which will benefit from further evaluation.
Third, we offer some novel back-of-the-envelope calculations for the cost-effectiveness of several interventions to treat pain. We conclude - in agreement with Sharma et al. (2020) - that providing opioids for terminal pain and drugs for migraines are potentially cost-effective interventions. We add an analysis suggesting that psychotherapy for chronic pain could be moderately cost-effective if it can be deployed in ways that reduce costs (task-shifted, grouped, and/or digital), although we doubt it would be as cost-effective as psychotherapy for depression. We also present other interventions which we are more uncertain about but we think are worth researching further.
There are many interventions we were unable to review. Reviewing the medical literature on pain was more time intensive than for our other projects because most meta-analyses evaluated their evidence as “moderate to low” quality. Furthermore, our subjective judgement was that these meta-analyses were of lower quality than the work we typically review from the fields of economics, psychology, and global health.
The most valuable directions for further cause prioritisation research are (1) narrowing our substantial uncertainty about the conversion rates between pain scores and SWB measures, and (2) investigating the potential of advocacy campaigns to increase access to opioids.
Outline
In Section 1 we define pain and present how it is measured.
In Section 2 we explore the relationship between pain and subjective wellbeing.
In Section 3 we model the scale and severity of chronic pain in subjective wellbeing terms.
In Section 4 we present potential interventions to treat pain.
In Section 5 we present our recommendations for future research and conclude with the key takeaways from the report.
Notes
- This report focuses on impact in terms of WELLBYs. One WELLBY is a 1 point change in life satisfaction for one year (or any equivalent combination of change in life satisfaction and time). In some cases, we convert results in standard deviations of life satisfaction to WELLBYs using a 2 point standard deviation on 0-10 life satisfaction scales (i.e., 1 SD change is the equivalent of 2 point changes on a 0-10 life satisfaction scale). This naive conversion is based on estimates from large scale data sets like the World Happiness Reports. See our post on the WELLBY method for more details.
- The shallowness of this investigation means (1) we include more guesses and uncertainty in our models, (2) we couldn’t always conduct the most detailed or complex analyses, (3) we might have missed some data, and (4) we take some findings at face value.
- Our calculations and data extraction can be found in this spreadsheet and GitHub repository.
1. What is pain?
We present some definitions and models of pain, discuss the most common types of pain, and explain how pain is measured.
1.1 Definitions and models of pain
According to the International Association for the Study of Pain (Raja et al., 2020, Box 2), pain is “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”. Therefore, we refer to pain as an unpleasant feeling (negative affect) related to actual or potential physical damage[3]. This unpleasant experience does not reduce to nociception - the sensory detection of physical harm - but needs a negative appraisal or emotion. Pain is influenced by multiple factors outside of sensory detection. Pain would be simpler to treat if it was merely nociception, but that is not the case. More recent and empirically-grounded models of pain suggest that many factors influence pain, and thus, there are different treatment possibilities for pain.
The experience of pain usually starts with nociception, where nociceptors (sensory neurons) detect some form of threat or tissue damage and send signals to the dorsal horn in the spinal cord. From there, signals are transmitted to the brain stem and further regions of the brain where they are processed into the unpleasant experience (Gatchel et al., 2007; Scholz & Woolf, 2002). Pain can also be neuropathic; pain due to damage to the nervous system itself (Scholz & Woolf, 2002).
Early models and biomedical approaches to pain have been criticised for interpreting pain solely in terms of nociception (Gatchel et al., 2007). Whilst sensory detection of physical damage is important, the quality that makes pain ‘painful’ is the unpleasantness. Namely, nociception without unpleasantness is not pain (Melzack & Casey, 1968). Melzack and Wall’s (1965) Gate Control Model, broadened the representation of pain by including top-down processes (cognition, experience, emotion, etc.) that can inhibit or excite signals in the dorsal horn and thereby modulate the experience of pain. This conception is broadened further with the current biopsychosocial model of pain (Gatchel et al., 2007), where multiple biological (genetics, different sensory factors, etc.), psychological (cognition, emotion, and behaviour), and social (social support, cultural beliefs about pain and the causes of pain, etc.) factors interact to create the experience of pain[4].
This representation of pain - an unpleasant experience that is more than the detection of physical damage and is influenced by many different factors - is important because including the role of psychological and social factors in a model of pain improves our understanding of the determinants, scale, and severity of pain. Additionally, it suggests potential treatments such as psychology-based approaches, that narrower models of pain would eschew. Furthermore, beliefs that pain must be directly related to bodily damage have caused people with conditions that do not conform to this erroneous model to receive inferior treatment (Foreman, 2014; Kerr & McRobbie, 2021).
1.2 Types of pain
We distinguish between two types of pain: chronic and acute. According to the International Association for the Study of Pain, chronic pain is pain “that persists or recurs for more than 3 months” (Treede et al., 2019, p. 19). It is further classified as chronic primary pain for conditions where the chronic pain is the disease in of itself, such as non-specific low-back pain, fibromyalgia, complex regional pain syndrome, migraines, irritable bowel syndrome, etc. Pain is classified as chronic secondary pain when the pain is a symptom of an underlying disease[5]. This chronic pain definition includes chronic cancer pain and chronic noncancer pain. Overall, a wide range of conditions is covered by this definition.
Conversely, acute pain is pain that lasts for less than 3 months, often for a much shorter time. This covers pain from stubbing one’s toe to excruciating pains from wounds (falls, bullets, animal bites, torture, etc.), as well as pain during surgery. Clearly, we want to avoid terrible acute pain. However, the range of acute experiences makes it difficult to evaluate in terms of a cause area. The issue is that we presumably want to prevent the most intense types of acute pain but we are unsure how well subjective scales capture the badness of extreme (and presumably unexpectedly bad) states of pain, as people might discover new depths of pain (what they thought was a 10/10 pain was actually only 8/10). This also poses questions of whether the simple integration of painful experiences over their duration is appropriate, but this is beyond the scope of this report. Consequently, our report focuses on chronic pain where we think, due to its duration, most of the suffering due to pain stems from.
1.3 Measuring pain
Many instruments attempt to measure pain (Bendinger et al., 2016). Unidimensional measures ask for self-reports of pain intensity: how much pain one is in. This is often, but not always, done with 0-10 scales (0 representing no pain and 10 representing the worst pain), such as visual analogue scales (0-10 cm) or numeric rating scales (0-10 points; Karcioglu et al., 2018). There are also verbal rating scales (e.g., ‘mild, moderate, extreme’) or even scales with faces ‘expressing’ different levels of pain (Karcioglu et al., 2018).
There are two common multidimensional scales for pain (with long and short forms). The McGill Pain Questionnaire (Melzack et al., 1975) includes: an illustration of a human body to indicate where the pain is experienced, selecting words that describe the pain sensation, items about how pain changes with time, and pain intensity items. The Brief Pain Inventory (Cleeland, 1991) includes: pain intensity items, a body illustration for reference, questions about pain relief, and items about aspects of life that pain has disrupted.
There are also multidimensional scales for specific conditions (e.g., neuropathic pain). Additionally, there is a grading measure for chronic pain that includes multiple items about pain intensity and disability (von Korff et al., 1992).
2. How pain relates to subjective wellbeing
In this section, we attempt to explain and quantify the badness of pain in terms of subjective wellbeing (SWB). First, we present reasoning about the conceptual relationship between pain and different accounts of subjective wellbeing. Then, we present our survey of the empirical literature relating pain and SWB. Our ultimate goal is to obtain a conversion rate between responses to pain measures and responses to SWB measures; namely, we want to be able to predict SWB changes when we only know changes to pain scores. We find two very different conversion rates (0.1 and 1) which is problematic because they suggest different priorities concerning pain interventions.
2.1 Reasoning about the relationship between pain and SWB
There are two main categories of measures and theories of subjective wellbeing: hedonist and desire/satisfactionist[6].
Hedonist theories claim that wellbeing is the balance of unpleasant and pleasant experiences. When combined, these constitute how happy someone is. The subjective wellbeing measures that most closely correspond to this hedonistic conception are questions that ask about how someone feels, how happy they are, or their positive and negative affective states. Pain and hedonia could, at least in theory, come apart: we can imagine someone saying they have lots of physical pain but are nevertheless in a good mood, or that they are in a bad mood but feel no pain.
Satisfactionist or desire theories claim that wellbeing is determined by the satisfaction of one’s desires. The SWB questions that most closely correspond to desire theories are those that ask how satisfied someone is with their life[7]. Equally, we can imagine someone being in pain but this barely affects their judgement of their life, or someone having poor life satisfaction but no pain.
How closely related measures of pain and SWB will be in practice depends on what’s being asked and how respondents interpret it. The measures of pain we encounter most often in the literature are unidimensional questions about how intense pain is or how much pain is experienced (see Section 1.3). We believe it is plausible that respondents answer these pain questions by thinking of pain as unpleasantness with a physical location. We also think it is possible that people use pain to refer to all unpleasantness they are feeling. Similarly, people may refer to negative affect as solely unpleasantness with a psychological origin (e.g., sadness due to bereavement for example) or to all unpleasantness. Note that we use affect as synonymous with the pleasantness or emotional valence of an experience[8]. We can use this framework to make predictions about the empirical relationships that would most closely conform to these understandings (see Table 1). We will see in Section 2.2 that measures of pain and measures of negative affect do not seem to be highly correlated.
Table 1: Predictions about the strength of the pain and negative affect relationship
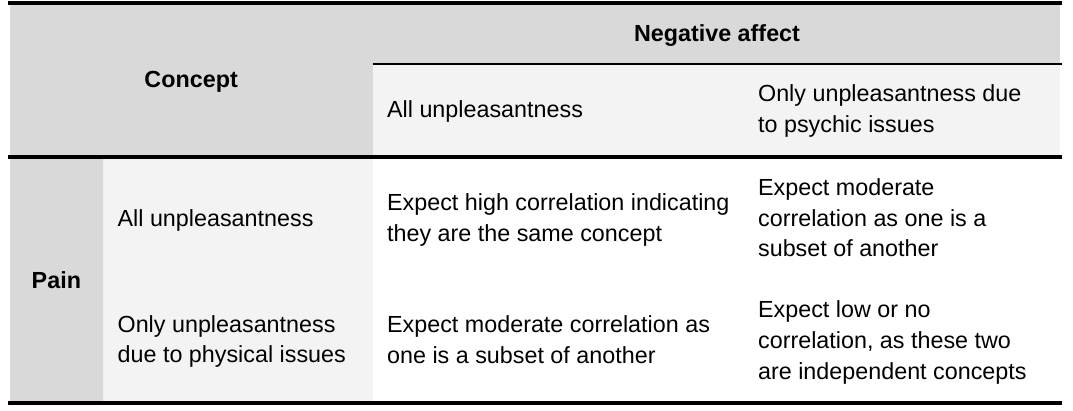
Importantly, there are also instrumental ways in which pain and its cause(s) can affect subjective wellbeing (Kahane, 2016). Pain and its cause(s) can cause disability or loss of function (which can incur social and economic consequences), preoccupy the mind, or lead to mental health problems[9]. One’s pain can also burden others: by spreading through empathy, by needing others to help, or by lowering one’s social and economic participation.
2.2 Empirical relationship between pain and SWB
Our aim was to quantify a relationship between measures of pain and SWB, but our analysis was inconclusive, primarily because we are uncertain which method best generalises. We found a few useful studies but no ideal study where pain measured on a 0-10 scale is regressed on several SWB measures (negative affect, positive affect, life satisfaction, happiness, and affective mental health). We estimate that a 1 unit decrease in pain could predict between a 0.1 to a 1 point increase in SWB. Note that the differences in conversion rates may be attributable to differences in the method but also the different types of SWB measures used in each (one method relates to life satisfaction whilst the other is in negative affect and affective mental health). Throughout, we consider issues with the current data that will need to be addressed with more research and evaluations that must be conducted in this domain.
2.2.1 Estimating the conversion rate between pain and SWB
As we’ve stated previously, we want to predict SWB changes when we only know changes to pain scores. An obvious place to start is by looking at correlations. Correlations between pain and various measures of SWB range between 0.2 and 0.3. See Table 2 below for sample-size weighted averages of correlations from different studies.
Table 2: Average (sample-size weighted) correlations between SWB and measures of pain intensity
| SWB measure | correlation | sample size | studies |
|---|---|---|---|
| life satisfaction | -0.31 | 813 | Furrer et al., 2017; Karadag Arli et al., 2017; Sturgeon et al., 2017 |
| positive affect | -0.25 | 4,817 | |
| negative affect | 0.24 | 996 | |
| depression (affective mental health) | 0.33 | 35,000 | Gerdle et al., 2019 |
This table conveys two important points. First, to our surprise, life satisfaction is (slightly) more strongly correlated to pain intensity than measures of positive or negative affect[10]. Second, these correlations are modest. This suggests to us that pain measures capture an experience that is related—but distinct—from what is captured by SWB measures. This casts doubt on the idea that people consider pain and negative affect to be the same concept. We think that the likeliest explanation for this is that people think of pain as “hurting”, so someone can hurt but also be in a good mood overall.
Because the weak correlations between pain and SWB measures indicate that pain isn’t measuring the same thing as negative affect, then we need to look elsewhere to quantify a conversion factor between SWB and pain measures. Correlations tell us about the noisiness of the relationship between two variables (their covariance), not the magnitude of that relationship. To assess how many units of SWB will change when pain changes by a unit we need either (a) regression models or (b) the ratio of the impacts of pain alleviation on both pain and SWB outcomes as measured in randomised controlled trials (RCTs)[11]. We present these methods below, which lead to very different results.
Estimating the conversion rate from regressions
We could not find the ideal regression study we would want, using 0-10 pain measures with 0-10 SWB measures. Instead, we find studies relating smaller pain scales with verbal labels to life satisfaction measures. Here, we look at how going from the minimum on these scales (‘no pain’) to the maximum (‘extreme pain’) relates to life satisfaction.
Some studies compare the EQ-5D health questionnaire, which asks respondents if they have no pain, moderate pain, or extreme pain. These studies find that having ‘extreme pain’ (versus ‘no pain’) in the EQ-5D is associated with losses of 0.5 (Graham et al., 2010), 0.7 (Mukuria & Brazier, 2013), or 1.3 life satisfaction points (Dolan & Metcalfe, 2012[12]). Birkjær et al. (2020) found similar results with a different scale: going from ‘mild’ to ‘extreme’ pain (on a ‘mild, moderate, extreme’ scale) is associated with a loss of 1 life satisfaction point. For context, being ‘extremely anxious or depressed’ is associated with a loss of 3.6 life satisfaction points in Dolan and Metcalfe (2012).
One issue is that we are unsure whether these measures encourage respondents to report their pain cardinally; namely, is the difference between ‘no pain’ and ‘moderate’ pain the same as that between ‘moderate’ and ‘extreme’ pain?
Another issue is how we might convert going from ‘no pain’ to ‘extreme pain’, and its effect on life satisfaction, into a change in pain on a 0-10 scale. We consider going from ‘no pain’ to ‘extreme pain’ to be going from 0 to 10 on a pain scale, an 11-point change. Hence, we take the higher end of these estimates, 1.3 points, and divide by 11, to calculate that a one unit change on a 0-10 measure of pain will lead to a 0.12 unit decrease in life satisfaction[13].
Estimating the conversion rate from outcome ratios in RCTs
In our analysis of the cost-effectiveness of psychology-based therapies for chronic pain (see Section 4), we find that these therapies reduce pain by 0.24 SDs and improve SWB (in negative affect and affective mental health measures combined) by 0.26 SDs. Hence, naively, the effect of the therapies on SWB was 109% that of the effect on pain reduction (i.e., a 1-unit decrease in pain represents a 1.09-unit decrease in negative affect and affective mental health measures).
This is a much bigger conversion rate than when using regressions. Here the intervention had a similar impact on pain intensity and SWB, but perhaps that’s because psychotherapy for pain improves SWB through channels beyond reducing pain intensity. Also note that part of this difference between methods may be due to the regressions only measuring life satisfaction whereas these RCT studies of psychology-based therapies measured negative affect and affective mental health.
Estimating the conversion rate like this is probably limited to predicting the effect on SWB of interventions analogous to psychotherapy. We would not be surprised if we found a different ratio of effects with a pharmacological intervention. Unfortunately, we didn’t find another intervention where both pain and SWB were measured. However, given the size of the pain literature, we expect that more evidence is out there, but it may not be easy to find.
Discussing the estimates
The two conversion rates we estimated indicate that a 1-unit decrease in pain seems to predict a 0.12-unit decrease in life satisfaction (from linear regressions), but a 1.09-unit decrease in emotional affect (from the effects of psychology-based therapies), on an assumed 0-10 scale. This is an order of magnitude difference and may be decisive for prioritisation. Because we can’t separate the SWB measures used in each method, we’re unsure whether it’s the method or the measures driving the difference. We speculate that it’s likely to be attributable to the methods because, based on our experience of reviewing interventions, the choice of SWB measure typically changes the results far less than a 10x difference.
The different conversion rates will imply vastly different cost-effectiveness estimates. In Section 4.1.1 and Section 4.1.2, we present back-of-the-envelope calculations for opioid treatments for palliative pain. The cost-effectiveness of advocacy for opioids is 18 times as cost-effective as GiveDirectly for a 0.12-point conversion rate, but 168 times as cost-effective as GiveDirectly for a 1.09-point conversion rate. This is because the effect of opioid treatments for palliative care ranges from 2 to 21 times as cost-effective as GiveDirectly depending on these conversion rates. In Section 4, we present the average effect of these interventions, where we use the naive average of the two estimated conversion rates: a 0.61 conversion rate.
2.2.2 Other issues with estimating a conversion rate
We believe that with access to the data from some of these studies, or access to a panel dataset that contains both pain intensity (0-10) and SWB measures, we could improve our estimates. But lack of data and analyses aren’t our only worries.
There is a potential issue of bidirectionality: high pain can reduce SWB, but low SWB could also exacerbate pain. For example, there is tentative evidence in a cohort study that pain can be predicted by life satisfaction two years earlier (Larsson et al., 2019).
Whilst we have good reason to believe SWB scales satisfy cardinality in general and that respondents use them linearly (i.e., a one-point change in the SWB experienced is reported as a one-point change in the measure; Plant, 2020b), there are still some potential scale use issues in the pain-SWB relationship. Some research by Myles and colleagues suggests that patients use pain intensity scales in a linear manner (Myles, 1999; Myles & Urquhart, 2005), but this is debated. Hartmannsgruber et al. (2020) suggested other methodologies should be used. Pesudovs et al. (2005) argued that Myles and colleagues are only looking at average use when the majority of usage should be considered instead. Kersten et al. (2014) have found that other analysis techniques challenge the linearity of the scales. We are also concerned with whether pain reports hold well for the worst experiences and how well SWB measures can capture these (e.g., Emilsson, 2019, mentions how extreme pain experiences might be units of magnitudes more severe than typical pain experience). Our concern is that, for subjective scales in general, the bounds are based on people’s predictions about what is reasonably possible. The worst experiences are by definition outliers, and likelier to fall below the lower bound of what people imagine. For example, what one might imagine reporting as a 10/10 pain experience for now (e.g., breaking a foot) might update when they experience more extreme pain (e.g., being tortured). In the future, we aim to explore the suitability of SWB to measure some of the worst experiences.
In this section, we attempted to quantify the relationship between SWB and pain, but our conclusion varies dramatically depending on the method we choose. The next step in exploring the cause area is quantifying the burden of pain on SWB through common pain conditions.
3. The scale and severity of chronic pain
In this section, we briefly survey the scale and severity of noncancer chronic pain in terms of subjective wellbeing (SWB).
Studies suggest that the prevalence of any form of chronic pain is around 20% of the population (sometimes more) in both high-income countries (Breivik et al., 2012; Dahlhamer et al., 2018; Fayaz et al., 2016; Henderson et al., 2013) and low- and middle-income countries (Jackson et al., 2015; Liao et al., 2022; Sá et al. 2019). With a naive calculation, this represents 1.55 billion people out of the 7.76 billion people in the world[14]. Some of the most prevalent forms of chronic pain are headache-based (e.g., migraines and tension headaches) and musculoskeletal (e.g., lower back pain and arthritis). Furthermore, the percentage of people with chronic headache-based or musculoskeletal conditions has been slightly increasing from 1990 to 2019 (GBD, 2019). Zajacova et al. (2021) estimated that the proportion of adults experiencing pain in the USA has increased between 2002-2018. Chronic pain is a very large-scale problem.
What about the severity of chronic pain? As we mentioned in Section 2, it is difficult to model a dose-response relationship between pain and SWB, but SWB can be useful in capturing all the negative effects of pain and the condition that causes it (comorbidities with mental health issues, loss of function, etc.). We conducted a non-exhaustive search of the literature for studies relating chronic pain (in general or the most common conditions) and SWB. We find that having a chronic pain condition is associated with a -0.11 (95% CI: -0.18, -0.04) SDs (13 effect sizes, 490,460 observations)[15] reduction in life satisfaction. Assuming this effect lasts for the whole of a year with chronic pain, it would be a loss of -0.22 WELLBYs. Considering the 1.55 billion people with chronic pain, this represents a loss of 340 million WELLBYs, each year, globally.
This suggests that having chronic pain reduces life satisfaction by ~0.2 points on a 0-10 scale. This seems small. Consider, for example, that in Birkjær et al. (2020), having arthritis (which can cause chronic pain) reduced life satisfaction by ~0.4 points whilst depression reduced life satisfaction by ~1.3 and unemployment reduced life satisfaction by ~0.6.
We have reasons to believe this estimate might not fully capture the burden of chronic pain:
- It does not include household spillovers. It seems likely that experiencing chronic pain may negatively impact other members of the household.
- Studies that use controls for economic, social, or mental health factors in estimates of the effect of chronic pain are potentially underestimating the effect because chronic pain is likely to cause economic, social, and mental health problems (so the effect of these problems should be attributed to the chronic pain). For example, Breivik et al. (2012) found that 19% of respondents had lost their job because of their pain and 21% had been diagnosed with depression because of their pain.
- The opposite problem is also an issue in our calculations. The studies we found cannot give perfect causal accounts of the effect of chronic pain on SWB, because there might be unobserved factors that cause both chronic pain and low SWB.
- These are averages that might not be fully capturing the burden of chronic pain for those who have the worst instances of chronic pain.
- As we saw in Section 2.2, psychotherapy can improve the SWB of people with chronic pain by 0.26 SDs, which is more than the 0.11 SDs loss in SWB we find here. However, this is only a weak argument because, as we mentioned, this effect was measured with negative affect and affective mental health measures, not life satisfaction. The higher effect of treatment could also be because treatments are given to people with worse chronic pain than average.
An important dimension for determining the burden of chronic pain in SWB is the long-term trajectory of chronic pain and SWB consequences. Namely, how long does the chronic pain last and how does its effect on SWB change over time? An important question within this is to what extent people ‘adapt’ to having chronic pain (i.e., the effect on their SWB subsides and they return to their original SWB levels before they developed chronic pain). We did not have time to explore this question. However, the fact that pain treatments reduce pain and improve SWB (see Section 2.2) suggests that patients do not strongly adapt to chronic pain because they need treatment to alleviate the pain and its effect on SWB (although, the improvement in SWB could also occur through other pathways). Furthermore, consequences of chronic pain such as losing a job or developing depression (Breivik et al., 2012) are consequences that people do not substantially adapt to (Clark et al., 2018).
4. Treating pain
Numerous studies analyse the effects of pain treatments. Searching “pain” on the Cochrane database of systematic reviews yields thousands of reviews. Most reviews we read conclude that the quality of evidence for treatments tends to be ‘moderate’ to ‘very low’.
The effectiveness of an intervention depends on the type or source of pain it aims to treat. This produces a profusion of possible interventions to alleviate pain. Pain management is not a ‘one-size-fits-all’ process (Korwisi et al., 2021). Whilst some treatments (e.g., CBT, opioids) may be proposed for a range of conditions, their efficacy can vary considerably between conditions and individuals. While this is typically the case with the effect of interventions, it appears especially true with pain. For example, drugs can reduce pain on average, but not every patient (often fewer than 50% in trials) benefits from them (Moore et al., 2013)[16]. This means that while we evaluate certain treatment options in general (e.g., CBT), we acknowledge that practitioners will have to tailor recommended treatments according to the specific pain burden of their patients. Considering the size of the literature, a comprehensive evaluation was far beyond the scope of this report. The interventions we present, and the research behind them, only scratches the surface of the possibilities.
The rest of this section is broken into two parts. First, we discuss the interventions for which we provide back-of-the-envelope calculations (BOTECs) that, whilst speculative, update us about the cost-effectiveness of pain treatments. Then, we discuss some interventions that are much more uncertain and require further research before informing us. In both cases, we order interventions according to our best guesses of their cost-effectiveness. Our guesses are based on our reading of the literature and anchored around the BOTECs we conducted. We categorise each intervention according to several factors. First, we classify an intervention by its type, which is either directly deploying the intervention or advocating for a change in laws or practice. We then note the class of pain that the intervention targets. Next, we briefly describe the intervention we are imagining. Finally, for those interventions we conducted BOTECs for, we show our estimates of their cost-effectiveness (as multiples of GiveDirectly cash transfers[17]).
4.1 BOTECs of interventions to decrease pain in LMICs
Many potentially effective ways to treat pain are undersupplied in low and middle-income countries (LMICs), where we think it will be cheaper to alleviate pain. We only had time to perform four shallow BOTECs of intervention cost-effectiveness, which we present in Table 3 in order of estimated cost-effectiveness.
Table 3: Pain-reducing interventions in order of guesstimated cost-effectiveness
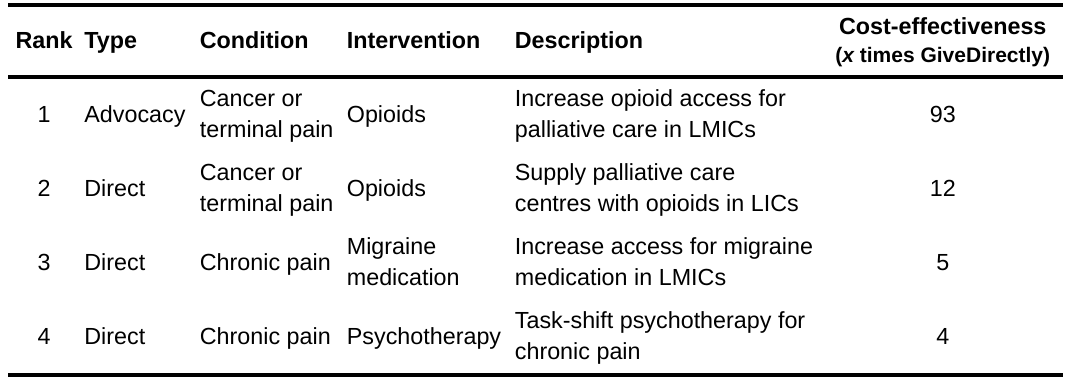
We propose three main types of interventions.
First, improving treatment of cancer or terminal/palliative pain in LMICs, especially with opioids. These seem to be the most promising. Our assessments of interventions in this area are based on the previous work of Sharma et al. (2020) as well as an important report by Knaul et al. (2017). These works report that opioid treatments for palliative pain are effective but face barriers related to education regarding, supply of, and policy concerning opioids. Importantly, the reason we focus on palliative pain with opioid treatments is because this is an area where potential adverse effects of opioids, such as dependence, are less likely to be problematic than for chronic pain (although, as we mention in Section 5, more research on adverse effects is still important). Second, we consider drug treatments for migraines. Third, is the deployment of psychology-based therapies for chronic pain which are less cost-effective. We imagine these therapies as task-shifted (and potentially group-based) to reduce costs, like StrongMinds does for depression (WHO, 2008; McGuire & Plant, 2021). However, none of the chronic pain treatments are as cost-effective as increasing wellbeing as StrongMinds is by treating depression in LMICs (9-10 times GiveDirectly; McGuire et al., 2022).
As we have seen in McGuire et al. (2022), household spillovers - how an intervention benefits people in the recipient’s household as well as the recipient - can affect the overall effect and cost-effectiveness of an intervention. We do not have data on household spillovers for pain treatments. However, we think it would be an inappropriate and nontrivial underestimate to not add, even speculatively, some form of spillover in our BOTECs. Previously, we calculated that 53% of the effects of psychotherapy for depression spillover to a non-recipient household member (McGuire et al., 2022). We use this 53% spillover ratio in our BOTECs and treat this as a prior we lack the confidence to update. Whilst this might not be the actual spillover of pain treatment, this is the most plausible estimate we can use as an informed prior. The other one available being the cash transfers spillover, which seems less plausible to us. The psychotherapy prior appears plausible because pain might affect the wellbeing of the household through similar mechanisms to those we present for depression (McGuire et al., 2022). These mechanisms are that the patient contributes less socially and economically to the household, and emotion contagion, where the unpleasantness of the patient's pain lowers the mood of other household members. Pain and depression are both health conditions that cause direct emotional suffering and indirect material losses. The overall benefits of an intervention are then calculated with:
effect on recipient + effect on recipient * spillover rate * non-recipient household size
We discuss each BOTEC in more detail in the following four sections.
4.1.1 Advocacy for increased access to opioids for palliative care in LMICs
Advocacy for decreasing the opioid treatment gap in LMICs appears potentially promising. In our BOTEC, we find it to be 93 times more cost-effective than GiveDirectly. But this estimate is extremely speculative because most inputs to this BOTEC are guesses. This is based on our BOTEC of the effect of opioid treatment for palliative pain presented in Section 4.1.2.
We imagine the population of a small low-income country or region of a populous country like India. We specify a population of 10 million. The calculation is sensitive to the population size inputted. Policy advocacy probably gets harder the larger the country, but we don’t include this in our model. For this population, we use the estimate from the WHO (2020) that ~1% of the population requires palliative care[18] and 95% of people requiring palliative care in LMICS have an unmet need for pain treatment (Knaul et al., 2017). Hence, 89,009 individuals will need palliative pain treatment.
Sharma et al. (2020) presents several case studies of advocacy appearing to succeed at increasing access to opioid-based palliative care after a decade of work. Based on our rough priors about policy advocacy and our impression of these cases we guesstimate there’s a probability of success of 10% each year for organisations like those presented (in part because the median success of these organisations seemed to occur after about a decade of work; 100 / 10 = 10% per year)[19]. This is a parameter that the results will be sensitive to, and is, as we said, a guess. Nevertheless, we find it useful to try to guesstimate the potential good this sort of intervention could do, because it is the one most often put forward as the most promising (as we will also argue), so we are setting the scene for more thorough evaluation. If we had more time we’d review the cases of improved paincare advocacy more systematically to sharpen our prediction of the likelihood of success.
If a reform was passed, we predict it would lead to a 33% increase in the share of opioid needs met. We take this from the case studies cited in Sharma et al. (2020) which led to a 12x increase in opioid access in Kerala and a 1.34x increase in Uganda. Averaging these two figures results in a 7x increase in the share of people with access. Since the number of people with access to effective palliative care is 5% (Knaul et al., 2017), increasing this by 7x would imply a 5% * 7 = 33 percentage point increase in the share of people with their opioid needs met.
From this, we estimate that amongst those needing opioids for palliative care (the 1% of the population - 100,000 - that needs opioids for palliative care in the arbitrary population of 10 million), 3,125 more would, in expectation, receive care attributable to advocacy. In Section 4.1.2, we estimate that for each person whose palliative pain is treated with opioids for a year, 5.6 WELLBYs are gained in that year. Counterfactually, we guess advocacy would bring reform forward by twenty years[20], adding 20 years where each year opioid palliative pain treatment is provided to 3,125 people. This results in an expected gain of 350,000 WELLBYs for effective advocacy to improve palliative care.
Lastly, we imagine that an organisation with a budget of $500,000 could achieve the aforementioned 10% probability of reform per year. To arrive at this we started with the budget for Lead Exposure Elimination Project when it advocated for the enforcement of lead paint regulations in Malawi ($100,000) but multiplied this by five (a guess) because we imagine the advocacy would need more people with country-specific knowledge and reputations.
Putting this together leads us to an estimate that advocacy for more opioid access is 93 times more cost-effective than GiveDirectly. Again, this estimate is very uncertain and crucially depends on our guess about the conversion rate between SWB and pain, as well as the likelihood of advocacy success. For example, if we use the lower or upper bound of the conversion rate, the cost-effectiveness ranges from 18 to 168 times as cost-effective as GiveDirectly.
4.1.2 Supply palliative care centres with opioids in LMICs
To understand the cost-effectiveness of interventions based on providing opioids in LMICS, we conduct a BOTEC of the benefits of opioids for treating palliative pain in LMICs. Here we imagine an intervention where a donor assists palliative care clinics to end their opioid shortages.
First, we need to estimate the SWB burden of extreme pain from untreated advanced cancer pain. We infer this indirectly using the conversion rates discussed in Section 2.1 because we did not find direct data relating palliative pain and SWB. Ideally, we want a study measuring SWB effects of palliative pain and the relief (and adverse effects) from opioid treatments.
We start by using the estimate from Sharma et al. (2020) for the pain severity of advanced cancer (the sort that involves palliative care) as 6.5 / 10 which would be reduced to 2 / 10 after opioids, a 4.5 point reduction. We then use the 0.61 point conversion score we presented in Section 2 to convert the results to SWB (in general, covering negative affect, affective mental health, and life satisfaction because the conversion rates we combined to get 0.61 cover all three of these measures). This suggests that opioids for advanced cancer improves SWB by 2.7 WELLBYs for each year of treatment.
As discussed earlier, we add a household spillover effect of 53% (like that we calculated for psychotherapy for depression; McGuire et al., 2022). We guess a household of two additional people. We assume a smaller household size than usual because household size tends to shrink with age (see Thomas et al., 2021; Rychtaříková & Akkerman, 2003). The overall benefit of treating palliative pain is 5.6 WELLBYs.
We imagine a fictitious charity that would supply opioids to palliative care centres with shortages in LMICs. It costs $34 to provide enough opioids to treat moderate to severe pain for a year (MSH, 2015). We add $10 to the per person cost to account for dealing with paperwork (this is a guess). Then to account for overhead costs we multiply the drug and paperwork costs by 1.5. This is in between a very lean and capital intensive NGO (such as GiveDirectly, where the factor would be close to 1) and a very labour intensive NGO (such as StrongMinds, where the factor would be close to 2). Altogether, this leads to a cost per person treated of $61 dollars per year. Overall, we predict this intervention to be 12 times as cost-effective as GiveDirectly.
Note that this estimate is very sensitive to our guess about the conversion rate between SWB and pain. For example, if we use the lower or upper bound of the conversion rate, the cost-effectiveness ranges from 2 to 21 times as cost-effective as GiveDirectly.
One concern we have, which we discussed in Section 2.2.2, is that subjective wellbeing scales may not be fit to measure extreme states like suffering from advanced cancer. Subjective wellbeing scales might break when life is unexpectedly bad. If this happens, it’s plausible that once someone experiences advanced cancer pain, their bottom of the scale shifts to become lower than it previously was. If such a shift were to occur, then our current calculation underestimates the cost-effectiveness of increasing opioid access because our conversion rate will not have accounted for this. This is an area we are unsure about and believe deserves further investigation.
4.1.3 Increase use of cheap migraine medication in LMICs
In this intervention, we imagine encouraging more effective self-medication with common over-the-counter analgesics. First, we think that there is a gap of self-treatment with cheap common medicines. A meta-analysis by Ghasemyani et al. (2022) finds that self-medication rates in Africa are 56% and in some countries it is lower, like in Ghana (25%) or Ethiopia (38%). We assume that this gap is also true for self-medication for migraines or headache disorders. At the least, we think there are rural areas with limited access to medicine that people could use to treat their own migraines. A published cost-effectiveness analysis of migraine treatment in LMICs assumed a coverage rate of 50%-80% for non-steroidal anti-inflammatory drugs (NSAIDs; Linde et al., 2015).
We obtain a meta-analytic average that having migraines reduces life satisfaction by -0.11 SDs (4 effect sizes, 123,112 observations)[21], or -0.22 WELLBYs. Common NSAIDs can eliminate most pain from migraines in half of all cases: aspirin, 52% (Kirthi et al., 2013) or ibuprofen, 57% (Rabbie et al., 2013). We assume that regular treatment with ibuprofen will half the burden of migraines and will thereby improve life satisfaction by 0.06 SD-years for every year the practice is maintained. We assume that once someone learns they can ease their migraines with ibuprofen, they’ll continue to do so for a decade. We aren’t sure about how long they’ll persist and whether it will continue to have the same effects. As we explained in Section 4.1, we add a household spillover rate of 53%, which increases the benefit of this intervention to 1.8 SD-years of life satisfaction (or 3.6 WELLBYs).
Ibuprofen is cheap. You can order 1,000 tablets online in Nigeria for $16. We imagine a fictitious charity that seeks to find migraine sufferers in LMICs and provide them with NSAIDs like ibuprofen, and educate them concerning the correct dosage. We assume the cost for an intervention addressing migraines would primarily be finding patients who haven’t already tried to self-medicate with ibuprofen. This seems feasible, as there are many places with very weak medical infrastructure or pharmaceutical access but we assume it will be relatively expensive to find someone to treat. We guess it will cost $50 to find someone to treat. We also guess that the overhead costs will be as much as variable costs (like StrongMinds), which would lead to a total cost per person treated of $104. As a result we estimate that the cost-effectiveness is 4.6 times GiveDirectly.
Honestly, we would be surprised if this intervention was anywhere near as simple as we make it out to be. The cost-effectiveness of this sort of intervention would depend on accessibility to NSAIDs and whether people would have purchased NSAIDs anyway. Whether individuals will rationally maximise their wellbeing (or health) is a whole other topic outside of the scope of this report. People in poverty and/or averse situations for their health don’t always purchase products that could benefit them immensely (in economic or health terms) such as fertiliser, anti-malaria bed nets, or deworming pills.
4.1.4 Task-shifted psychotherapy for chronic pain
Psychology-based therapies for chronic pain such as cognitive behavioural therapy (CBT; Knoerl et al., 2016), acceptance and commitment therapy (ACT; Hughes et al., 2017), mindfulness (Hilton et al., 2017), and positive psychology (Braunwalder et al., 2021), appear effective at reducing chronic pain. Note that the quality of the evidence is often considered ‘moderate to low’ (e.g., Williams et al. 2020). When one considers the psychological (cognitive and emotional) influences on pain (see Section 1), it makes sense that psychological solutions could help.
These therapies seek to help patients correct maladaptive beliefs, emotional reactions, and behaviours related to the pain. These emotion regulation techniques have been linked to reduced negative reactions to pain - and even reduced pain intensity - in experimental conditions (Jaén et al., 2021). One notable factor is how these therapies can reduce tendencies to catastrophize about the pain (e.g., Smeets et al., 2006; Niknejad et al., 2018). We saw in Section 1 that catastrophizing exacerbates pain. Additionally, these therapies would help with the additional burden of comorbid mental health problems that are prevalent in patients with chronic pain.
We conducted a quick BOTEC to estimate the cost-effectiveness of task-shifted psychotherapy for chronic pain. We obtain a meta-analytic average effect on SWB (negative affect and affective mental health measures combined) of psychology-based therapies based on seven meta-analyses[22]. Note that we are combining different therapies with different control conditions (treatment as usual, active, etc.), across different time spans (although usually the effect was measured immediately after post-treatment). Hence, this is a rough estimate. We find that these therapies improved SWB (negative affect and affective mental health measures combined) by 0.26 SDs (or 0.52 WELLBYs). Interestingly, we also find that they reduce pain by 0.24 SDs, suggesting that the effect on SWB and pain are similar. We combine this finding with the duration, household spillovers, and costs from our previous analysis of psychotherapy and StrongMinds (McGuire et al., 2022).
The assumption here is that psychology-based therapies for pain could be deployed to LMICs with a similar task-shifted group therapy model as StrongMinds. Providing the therapy in a group lowers the cost per person (and could also potentially reduce stigma about the condition by providing peers). Group therapy for pain does occur. For example, in a review of 25 trials of acceptance and commitment therapy, McCracken et al. (2022) found that 14 of them were group-based. Task-shifting means having non-specialists trained to deliver the specific therapy by specialists, which can save the costs of training fully-fledged therapists and help increase the number of providers (e.g., Raviola et al., 2019). In our brief search, we did not find examples of task-shifted psychotherapy aimed at pain. Note, we haven’t investigated whether task-shifting or group settings would moderate the effectiveness of psychology-based treatments for pain, but we assume at this stage that these methods would just make the therapies less costly without changing effectiveness.
We estimate an overall effect (for the household and individual combined) of 2.47 SD-years of SWB (4.94 WELLBYs). Imagining a charity that could deliver psychological-based pain therapy for $170 per person (the cost we estimated for StrongMinds), we estimate that psychology-based therapies for pain in LMICs would be 3.8 times as cost-effective as GiveDirectly. This is not as good as psychotherapy or StrongMinds for depression (McGuire et al., 2022). To be clear, this is because we use the average 0.26 SDs effect as the initial effect in our calculations, which is lower than the initial effect of 0.44 SDs we previously estimated for task-shifted psychotherapy in LMICs.
4.2 Interventions with less certain evidence or more research required
In this section, we discuss interventions to reduce pain that require more research before any BOTEC seems sensible. We introduce them in order of our view of their promise.
Table 4: Promising but even more speculative interventions to decrease pain
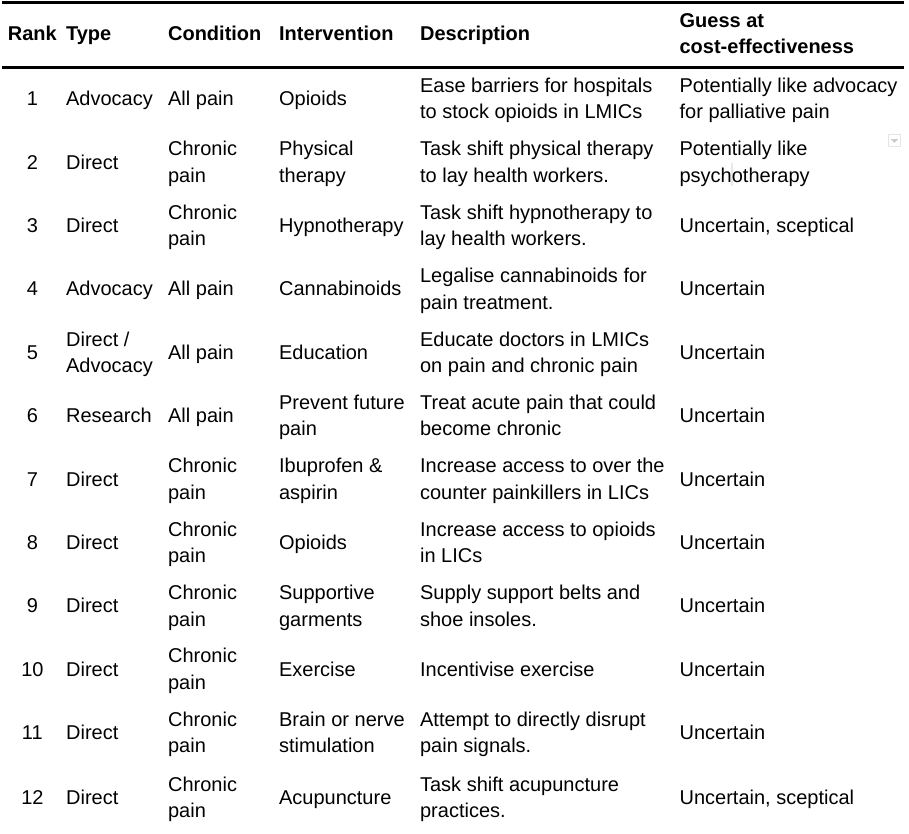
4.2.1 Advocacy for increased access to opioids for all hospitals in LMICs
Opioids are not only used to treat palliative pain, they can also be used for other pain experiences (e.g., surgery pain). There’s a huge, general, unmet need for opioids in LMICs (Knaul et al., 2017), so further advocacy for more access to opioids could be cost-effective. This intervention could be more effective than advocating solely for greater access to opioids for palliative care (Section 4.1.1) because it would presumably cover other areas where there is a clear need for opioids such as pain from acute traumatic injury.
However, we expect that expanding advocacy beyond palliative care would be a bigger push and raise the risks of negative effects coming from addiction and withdrawal. There is also more potential for political backlash or reputational harm (that could come from being accused of exporting the opioid crisis, for example). The higher risks for harm make us less confident about the prospects of general opioid access advocacy.
4.2.2 Task-shift physical therapy to lay health workers
Discussions with a doctor who specialises in physical therapy made us think that physiotherapy could be deployed with a group task-shifted approach. We think this might have a similar cost-effectiveness to psychotherapy because while it may be more effective at reducing pain intensity (an initial effect of 0.84 SDs; Marris et al., 2021), we expect it to be much more complicated and expensive to deploy as an intervention. Our understanding is that while it seems plausible that physiotherapy can be task-shifted, it will likely require more training than psychotherapy because a different exercise is required for each source of pain. This seems somewhat more intense than psychotherapy, and importantly, not easily done in a group setting – which is our other assumption about implementing psychotherapy.
4.2.3 Task-shift hypnotherapy to lay health workers
We are sceptical of the effects of hypnotherapy and unsure how scientific hypnotherapy is. We would want to explore the mechanisms of hypnotherapy in more detail before making any recommendations. It is possible that hypnotherapy functions in similar ways to psychology-based therapies (e.g., by helping with the reappraisal of pain). Our prior is sceptical, and we would be surprised if this was more cost-effective than psychotherapy.
Surprisingly, hypnotherapy works at reducing pain intensity in people with experimentally-induced (-0.74 SDs: Thompson et al., 2019) or clinical/chronic pain (-0.61 SDs: Milling et al., 2021; -0.42 SDs: Langlois et al., 2022). One long-term study found that a reduction in pain perception of 31% persisted for a year after hypnotherapy (Dumain et al., 2021). Notably, people who are more susceptible (as assessed by a common test) are much more affected by hypnotherapy. In Thompson et al. (2019), the pain reduction dropped from 42% in high susceptibility to 29% in medium susceptibility. One promising property of hypnotherapy is that it offers a high potential for being manualized if you can administer hypnotherapy simply by reading a script or playing a recording.
4.2.4 Legalise the use of cannabinoids for pain treatment
Cannabis/cannabinoid-based treatments have increased in popularity and the wave of legalisation in North America over the past decade promises the possibility of reform. Meta-analyses suggest that using cannabis reduces pain from 0.5 to 1.25 points on 0-10 scales (McDonagh et al., 2021; Mücke et al. 2018; Johal et al., 2020; Rabgay et al., 2020; Wang et al., 2021; Wong et al., 2020). Note that the quality of the evidence is often considered ‘low’ in these meta-analyses. This might be an approach that has fewer, but still nontrivial, adverse effects compared to other drug treatments like opioids. One risk, as mentioned in a small (N = 78) experience sampling study by Sznitman et al. (2021), is that patients want and use medical cannabis to treat their pain even when it does not significantly reduce their pain.
Successful advocacy for medical legalisation would also have spillovers on the criminal justice system (medical legislation would change how the drug is prosecuted). It may also have an impact on mental health. Black et al. (2019) found in 7 studies that cannabinoids decreased anxiety symptoms by -0.25 SDs and in 12 studies a small but non-significant decrease in depressive symptoms of -0.05 SDs.
Despite the potential benefits, advocacy may not be very cost-effective because (1) there already seems to be substantial political organisation around this topic, so more advocacy might not produce much more benefits, and (2) some countries might be more resistant to using medical cannabis than another less controversial therapy with similar benefits. We would need evidence that there are particularly promising advocacy opportunities for cannabinoids to update our view of their potential cost-effectiveness.
4.2.5 Educate doctors about pain treatment and chronic pain
Doctors in some high-income countries (HICs) get surprisingly little education on pain or pain management. For instance, 96% of medical schools in the USA and UK had no dedicated curriculum on pain (Shipton et al., 2018). Chronic pain education also seems to be lacking in HICs (Loeser & Schatman, 2017). Furthermore, the biomedical approach to treating pain has been criticised for its failures to treat chronic pain more holistically, neglecting the risks of certain treatments, the benefits of non-traditional treatments (such as psychology-based treatments), or sometimes plainly not providing pain relief (Lalkhen, 2022). We assume the issue would be as bad or worse in LMICs. Indeed, some research in Zimbabwe points to a gap in the teaching of chronic pain (Moyo & Madzimbamuto, 2019).
If we could cheaply develop workshops about pain, chronic pain, and cost-effective pain treatments, these could lead to lasting improvements in a doctor’s treatments of patients. This seems plausibly cost-effective. However, there are some important uncertainties left unanswered. We would need to know more about the doctors’ current knowledge and practice for pain relief and their openness to change. Would this actually work? How has this been tried before? It would strike us as a bit strange if an intervention to educate people who should be best placed to treat pain was cost-effective. We accept that pain treatment is suboptimal in many places and could be the practitioner's fault, but we would be surprised if doctors’ knowledge or attitudes was the biggest bottleneck in improving pain treatment.
4.2.6 Can we prevent chronic pain?
One important avenue for cost-effective interventions related to pain is to prevent pain in the first place. If someone does not develop chronic pain because of an intervention, then it has counterfactually prevented the SWB burden of having chronic pain.
For example, there is tentative evidence that physical activity/exercise can lower the risk of lower back pain (Shiri et al., 2018; Steffens et al., 2016; see also Sharma et al., 2020).
We would most like to see more research exploring how undertreated acute pain can become chronic pain. Acute pain, from surgery or traumas, can - because of neuroplasticity and changes in the processing of pain - lead to changed pain responses to the current nociceptive information and result in chronic pain (McGreevy et al., 2011). There are a range of risk factors for post-operative pain becoming chronic pain, including the pain related to the operation but also psychosocial factors, comorbidities, and surgery factors (McGreevy et al., 2011). There is some evidence that analgesia before and during the operation (i.e., before the tissue damage occurs) can reduce the risk of post-operative and chronic pain (McGreevy et al., 2011). Concerning treating postoperative pain with drugs, Carley et al. (2021) conducted a large meta-analysis of 110 studies of different drug treatments (ketamine, NSAIDs, etc.) for postoperative pain and concluded that the effects were small, with potential adverse effect risks, and that the evidence was insufficient to recommend clinical use to prevent chronic pain. The evidence on psychology-based interventions is also very limited with only three studies (Orenius et al., 2022).
Early-life noxious stimuli (injections, surgeries, etc.) for neonates (especially if ill or premature) have been shown to have potential adverse effects in development, such as increased sensitivity to pain (Williams & Lascelles, 2020)[23]. Pain relief is not always provided to neonates (Mathew & Mathew, 2003; Simons et al., 2003). Research also suggests that pain (Walker et al., 2010) or adverse experiences (abuse, trauma, etc.; Beal et al., 2020; Nelson et al., 2017) in childhood are related to pain and chronic pain in adulthood. These are complicated areas of study, but it suggests that improving early life can reduce the burden of pain later on and reap multiple benefits across time.
However, the causal modelling of how early experiences - whether in childhood or due to events like surgery - affect later pain is still in need of research. Open Philanthropy has made some research grants related to these topics (see here and here for example). This is a complex question, but it could reveal extremely important altruistic priorities.
4.2.7 Increase access to NSAIDs in LICs
Whilst weaker than opioids, non-steroidal anti-inflammatory drugs (NSAIDs) like aspirin or ibuprofen can still alleviate pain. We’re uncertain about whether they are accessible in low-income countries (LICs). We would like further research to clarify if there is any gap, and if so, why it exists. If NSAIDs appear undersupplied in LICs, closing that gap seems like an intervention worth considering.
4.2.8 Increase access to opioids for chronic pain in LICs
In Sections 4.1.1 and 4.1.2 we discussed using opioids to treat pain from advanced cancer or terminal illnesses. Using opioids for this purpose is widely accepted. But opioids are also often used to treat chronic pain (e.g., Deyo et al., 2011, found that 19% of chronic back pain patients in the USA were long-term users of opioids). Long-term use of opioids garnered controversy due to the increase in deaths attributed to opioids in North America, also known as the opioid crisis or epidemic (Shipton et al., 2018; Lyden et al., 2019; Rudd et al., 2016; Case & Deaton, 2020). There is heterogeneity in the findings concerning rates of opioid dependence in chronic pain patients - from less than 1% to about 30% - in part because of different definitions for ‘dependence’ (Voon et al., 2017).
Meta-analyses and reviews of opioid treatments for chronic pain do find moderate reductions in pain (Busse et al. 2018; Chou et al. 2022). In a meta-analysis of 96 studies (26,169 patients), Busse et al. (2018) found that opioids significantly reduced pain by 0.7 points (on a 0-10 scale). However, there is a lack of evidence concerning the effects of opioid treatments in the long-term (more than 12-16 weeks; Montgomery, 2020). Furthermore, opioids do not always seem to perform better at treating chronic pain than other non-opioid drugs such as NSAIDs or acetaminophen (Chaparro et al. 2013; Chou et al. 2022; Krebs et al., 2018). There are concerns about the potential adverse effects opioid treatments can create compared to placebo or even other non-opioid drugs, such as vomiting, constipation, or dependence (Chou et al. 2022; Els et al., 2017; Montgomery, 2020). There is also concern that patients who dropped out of opioid trials because of adverse effects are sometimes counted in ways that inflate the effectiveness of opioid treatments (Moore et al., 2012).
Before making any conclusions about the effect of opioid treatments for chronic pain we would want more SWB estimates of the positive and adverse effects of such treatments. We note that NICE (2021) recommends against opioid treatments and the CDC (2016, Box 1) states that “nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain”. Given the uncertainty and controversy surrounding this topic, we remain unsure about whether opioids for chronic pain are under or oversupplied. With further research, we would try and clarify this issue.
4.2.9 Supply support belts and shoe insoles for back pain in LMICs
Lower back pain is a problem for a lot of people (Sharma et al., 2020). There is a range of treatments that can have small benefits (including exercise and psychology-based therapies; Chou et al., 2016). However, our impression is that determining which would be a truly cost-effective approach for lower back pain needs more research (see also Sharma et al., 2020).
Huang et al. (2018) found that shoe insoles (5 studies) and back belts (6 studies) had small non-significant effects on pain intensity (-0.22 and -0.06 SD reductions, respectively). While this is not very promising in of itself, we think it is worth further research. If permanently reducing back pain could be as simple as shoe insoles, then that seems like a potential for-profit intervention.
4.2.10 Incentivise exercise
A meta-analysis by Huang et al. (2018) summarised 8 studies to find that exercise significantly reduces the intensity of chronic pain by -0.29 SDs (see also Chou et al., 2016). Plus, exercise might help prevent some forms of chronic pain (see Section 4.2.6). NICE (2021) recommends exercise and physical activity for the treatment of chronic pain. On one hand, this is promising because anyone can exercise. On the other hand, it’s unclear how this could be implemented as an intervention in low- or middle-income countries. A large share of the population still works in manual labour, so advocating exercise may come across as tone-deaf, to put it lightly. Further, it’s unclear how to incentivize exercise or even if incentives would work, our prior view is that changing human behaviour in this way is difficult.
4.2.11 Brain or nerve stimulation to reduce pain
There are several interventions that aim to alleviate pain by directly affecting the nerves or brain regions that generate pain.
There are non-invasive methods in this domain. One RCT, reviewed in Gibson et al. (2017), found that transcutaneous electrical nerve stimulation decreased the pain intensity of people with neuropathic pain -1.6 SDs. In a meta-analysis of 27 studies, O'Connell et al. (2018) reviewed several other methods of non-invasive brain stimulation and found reductions in pain intensity between -0.22 and -0.43 SDs.
Spinal cord stimulation involves implementing a device in the body (which is more invasive) that sends small electrical impulses to the spinal cord. This can produce pain relief for a range of chronic pain conditions. In a systematic review, Baranidharan et al. (2021) concluded that the treatment is safe and can produce 48-64% reduction in pain for 46–76% of patients beyond 12 months of follow-up. However, this treatment seems to be expensive, with costs beyond $10,000 per person (Lalkhen, 2022; NICE, 2019; Ontario Health, 2020).
We are also intrigued by the possibility that deep brain stimulation could eliminate pain and induce intense pleasure[24] in palliative care patients. Bittar et al. (2005) found that deep brain stimulation provided lasting pain relief for 58% of patients with previously hard-to-treat pain. A 77% reduction in cluster headache severity was found from deep brain stimulation in Nowacki et al. (2020).
4.2.12 Task-shift acupuncture practices
National Institute for Health and Care Excellence (NICE, 2021) recommends acupuncture as a potential treatment for chronic pain. However, acupuncture and related research has been criticised (Colquhoun & Novella, 2013) and we have reasons to be sceptical of it as a treatment. Whilst meta-analyses of acupuncture suggest it might be an effective treatment for chronic pain (MacPherson et al., 2017; Vickers et al., 2018; Zheng & Zhou, 2022), the results become small, more uncertain, and often non-significant when compared to sham controls. We’d require much more evidence of its effectiveness to update us towards considering it as anything other than placebo.
5. Recommendations for future research
We recommend more research to improve and expand on this report. We present the topics in order of the most informative and time-efficient questions to answer.
- As we discussed in Sections 2, 3, and 4, we’re very uncertain about the relationship between pain and SWB and the plausible values this could take would dramatically vary the cost-effectiveness of pain-reducing interventions. We need more research to clarify the relationship between pain and SWB. Specifically, we think it’s possible to gather more data that allows us to regress the effect of pain on various measures of subjective wellbeing (instead of just life satisfaction). We’re optimistic that we can find more pain-reducing interventions that record their impact with both pain and SWB measures. The goal would be to have an accurate conversion rate between pain and (different measures of) SWB so that data that reported changes in pain outcomes can be ‘converted’ to SWB.
- We also want to know if SWB measures can assess extreme conditions (e.g., extreme pain from advanced cancer) and about the potential household spillovers of being in pain and pain treatments.
- Advocating for, and providing, opioid-based treatments for palliative pain in LMICs seem to be the most promising interventions concerning pain. We recommend more scrutiny of the evidence that feeds into this model. If we had more time we would prioritise (a) trying to find additional ways to estimate the SWB burden of advanced cancer, and (b) systematically collecting case studies of palliative care reform to better estimate the likelihood of advocacy success (see Sharma et al., 2020 or OPIS for example).
- For chronic pain, we find that providing drugs for migraines or task-shifted psychology-based therapies might be moderately cost-effective. More research on the cost of deploying these interventions could easily clarify their potential cost-effectiveness. We think it’s also worth exploring different cost-reducing implementations. For example, some treatments like psychotherapy could be delivered digitally. Eccleston et al. (2020) mention the eCentreClinic’s online courses on chronic conditions and pain. This can be time-consuming but will clarify the cost-effectiveness of multiple treatments.
- A valuable, but Herculean, project would be to construct a ‘Global Burden of Pain’ in the form of a report or database that has a prevalence measure, pain measure, SWB measure, and potential treatment estimates for a range of acute, terminal, and chronic conditions. We were unable to even find a study or database that reports a measure of pain intensity across a range of conditions. What is available are DALY estimates for some conditions in the Global Burden of Disease. However, DALYs seem implausibly insensitive to pain. Sharma et al. (2020) pointed out that the DALY rankings for treated and untreated cancer are almost the same, which seems dubious.
In conclusion, pain alleviation is a wide topic, but one deserving more research. We think there’s likely to be some altruistic gold here. However, we are uncertain about the effort it will take to uncover it. We believe that further cause prioritisation research should address our substantial uncertainty about the conversion rates between pain scores and SWB measures and investigate the potential of advocacy campaigns to increase access to opioids.

- ^
Samuel Dupret and Joel McGuire contributed to the conceptualization, investigation, analysis, data curation, and writing (original as well as review and editing) of the project. Michael Plant contributed to the conceptualization, supervision, and writing (review and editing) of the project.
- ^
In the effective altruism community, chronic pain and access to pain relief have been proposed as potential cause areas and issues of extreme pain have also been mentioned here, here, and here. Peter Singer (2018) has written about the lack of access to opioids and Charity Entrepreneurship has also been looking into pain (we thank them for sharing some documents with us). Despite this work, there has been relatively little in-depth research or concerted effort to pilot potential interventions. Notable exceptions are the Organisation for the Prevention of Intense Suffering (OPIS) and Open Philanthropy’s grants for pain research.
- ^
When referring to the general label ‘pain’, some might include ‘psychological pain’, unpleasant feelings that do not originate from a region of the body (depression, social ties breaking, bereavement, etc.). While it makes sense to refer to pain as either physical or psychological, in this report we will follow the convention of using pain to refer primarily to pain related to the body.
- ^
Here are a few examples that illustrate that the source of pain is more complex than sensing damage. Soldiers often only notice wounds when the fighting is over. People can also experience pain in phantom limbs, limbs they no longer possess (Flor, 2002). There is a class of pain called ‘nociplastic’, where there is a pain experience despite no clear evidence of body damage. Nociplastic pain is present in chronic pain conditions such as fibromyalgia (Kosek et al. 2016; Raja et al., 2020). Context is also an important determinant of pain; for example, civilians and soldiers experience similar wounds differently (Beecher, 1959). Attention affects our experience of pain; more attention can increase pain, whilst less attention decreases pain (Wiech et al., 2008). Thus, distraction from the source of damage can reduce the experience of pain (Bascour-Sandoval et al., 2019; Gupta et al., 2018). Expectations of pain also play a role (Bingel et al., 2011); positive expectations can reduce pain (placebo effect) whilst negative experiences can increase pain (nocebo effect). Perceptions that we are in control of the pain reduces the pain experience (Wiech et al., 2006). Being in a good or bad mood can change how unpleasant pain is (Bushnell et al., 2013). Catastrophizing, that is, exaggerated negative appraisals and beliefs, can exacerbate pain (Covic et al., 2003; Sullivan et al., 2001).
- ^
“chronic cancer-related pain, chronic neuropathic pain, chronic secondary visceral pain, chronic posttraumatic and postsurgical pain, chronic secondary headache and orofacial pain, and chronic secondary musculoskeletal pain” (Treede et al., 2019, p. 19).
- ^
Eudaimonia (a sense of meaning and purpose) is sometimes proposed as a third theory of wellbeing.
- ^
See Plant (2020a) for an in-depth discussion of this relationship.
- ^
Definitions in psychology we have come across are more confused. They conflate affect with dissatisfaction or “self concept”.
- ^
The experience of pain and depression seem intertwined, exacerbating each other or making each other more likely (IsHak et al., 2018; Von Korff & Simon, 2018).
- ^
There is only one study that compared life satisfaction and negative affect within the same sample (Furrer et al., 2017), and there, the correlation between pain and negative affect (0.22) is larger than for pain and life satisfaction (-0.07). However, this study only has a small sample size of 96 respondents, so we interpret the average findings instead.
- ^
Other studies report the relationship between amounts of pain or pain conditions with percentages of people satisfied with their life (Silvermark et al., 2008) or of people with depression (Sudarisan et al., 2019). We cannot straightforwardly use this information to create a conversion estimate. This is because the studies are providing us with mean differences, which doesn’t inform us about how the rates of satisfaction or depression would differ if the levels of pain differed. The data is there to do the right analysis for our purposes in studies like this, but the authors did not conduct that analysis.
- ^
Dolan and Metcalfe (2012)’s results are initial on a 0-6 scale, which they then rescale to 0-1, which we rescale to 0-10 by multiplying by 10.
- ^
However, an issue here is that we are assuming people use the end points of a ‘no pain’ to ‘extreme pain’ scale in the same way as the 0 and 10 on a 0-10 scale. 0-10 pain scales are often introduced with labels, such as ‘no pain’ for 0 and ‘worst pain’ for 10. It is likely people use ‘no pain’ and 0 to be equivalent, however, whilst they might use ‘extreme pain’ for values of 10, they could also mean 7, 8, or 9. Namely, we would have underestimated the conversion factor (i.e., divided by too many points) if people report going from ‘no pain’ to ‘extreme pain’ to mean 0 to 7 instead of 0 to 10. If we assumed a more modest mapping where ‘extreme pain’ only means a 7 on a 0-10 pain scale, the conversion rate would be 1 to 0.16 instead of 0.12.
- ^
Value from the World Bank (2020).
- ^
Effects from 8 studies (Asgeirsdottir et al. 2017; Binder & Coad, 2013; Birkjær et al., 2020; Groot et al., 2004; Howley, 2017; McNamee & Mendolia, 2014, Ólafsdóttir et al., 2020; Powdthavee & van den Berg, 2011). See our data and analysis for more details.
- ^
Pain reduction is often reported according to two important factors: how much it reduces pain (where analgesic success is often defined as 30-50% reduction in pain) and how many patients experience this change (Borsook et al. 2018; Dworkin et al., 2008).
- ^
40 million people in LMICs, where the total population is about 3.3 billion.
- ^
Our heuristic for policy advocacy is that popular and obvious reforms for simple regulatory actions that have little opposition have the highest chances of success (20% - 100%). We think advocating to ban pesticides used to commit suicide or lead in paint are examples of this category. The next category are interventions that are still popular but may require more administrative action to implement or face a stauncher, more coordinated resistance (likelihood: 1% to 20%). We think opioids fit in this second category. It seems likely to be popular (albeit dependent on attitudes towards opioids), but seems like it could be relatively expensive for the government to administer.
- ^
Ideally, we would compare the change in a country's opioid access in two groups of countries: (a) countries with advocacy movements that achieved reform, and (b) countries without advocacy. Our reasoning for this is that many countries' incomes are increasing and we think better pain access will slowly follow wealth. We should get a better sense of the trend in the share of opioid neets met. Another element to consider is that in some LMICs like China, their population is ageing before they've become wealthy with well-funded welfare states. This will increase the burden of pain.
- ^
Effects are drawn from 4 studies (Asgeirsdottir et al. 2017; Binder & Coad, 2013; Groot et al., 2004; Powdthavee & van den Berg, 2011).
- ^
- ^
We thank Ulf Johansson for bringing this topic to our attention and providing references.
- ^
Quoting Ng (2020): “In humans, ‘patients who were having emotional or physical pain experienced such intense pleasure with stimulation that the pain was obliterated’ (Heath et al. 1968, p. 188). Scholars describe the feeling from brain stimulation as ‘super–pleasure or ‘supramaximal’ (Dror 2016.)”
NickLaing @ 2023-01-28T09:59 (+31)
First I think pain relief interventions have enormous potential to be high impact, and thanks for this analysis. I love your comments about DALYs being relatively insensitive to pain as well. From a practical perspective I'm not sure about some of your potential focus areas though so here are some coments.
As a doctor here in Uganda, my bet for the highest impact pain area might be undertreatment of chronic pain in older people with arthritis. Most people are subsistence farmers, and from age 40+ many (if not most) people get arthrits in their knees, ankles, and shoulders which causes a lot of pain and stops sleep This chronic arthritic pain is massively undertreated, despite simple analgesics making a big difference. People can't do everyday jobs, struggle to sleep and this often has negative synergies with mental illness too. Our health centers see loads of these patients focus on giving large enough amounts of paracetamol and ibuprofen which people can take at home when their pain is bad. The scale of this problem is massive, and it seems fairly neglected and tractable. This has overlap with NSAID access, but is not the same thing.
On access to NSAIDS, I don't think access is that bad in lower income countries. NSAIDs are in every drug shop in every village here, but the issue is with HOW they are given. Medical providers just don't give nearly enough pain relief especially for arthrits in older people. I feel like it's more of a prescription and understanding issue than an access issue. Of course they cost money too so there is some access problem.
You also have to be VERY careful about gastritis and peptic ulcers which are super common in LMICs but NSAIDS can cause or make worse. NSAIDs definitely shouldn't be given out like lollies that's for sure, but could be given out a lot more.
I like the opiods idea, although I think you might be undercosting them, and also perhaps underrating both the difficulty and the risk of harm of scaling them up. We did never have enough morphine at the hospital I used to work at which was pretty rough. I also don't really know what you mean by "palliative care centers" in low income countries maybe you could give a couple of examples (websites). Where these exist in low income countries, they are the realm of the rich, or run by NGOs who already have access to opiods. Or do you basically mean hospitals which have palliative care units? Hospitals use morphine yes, and access can definitely be improved. https://bmcpalliatcare.biomedcentral.com/articles/10.1186/s12904-022-00930-7
Migraines are not super common, difficult to diagnostically separate from other conditions, and specific drugs for them are very expensive. My fairly strong instinct is that can't imagine this ever being a high impact area of intervention.
I know these are only shallow investigations, but if you don't have access to experts on pain in LMIC countries (which probably exist, especially in the palliatve care field) I think you could potentially even save time early in your investigation process by talking to a few medical professionals in lower income countries to get more ideas and screen out a couple of these interventions.
JoelMcGuire @ 2023-01-30T19:46 (+9)
Nick,
This is super helpful feedback.
Do you have any ideas for how one would most cost-effectively treating arthritis pain in a low income country?
On access to NSAIDS, I don't think access is that bad in lower income countries. NSAIDs are in every drug shop in every village here, but the issue is with HOW they are given.
Okay, this is good to know. Ditto with the bit about gastritis and peptic ulcers.
I also don't really know what you mean by "palliative care centers" in low income countries
We weren't sure what type of facilities had the biggest supply problems that could be ameliorated, so we were unsure. Good to know palliative care units may have relatively more access problems.
Migraines are not super common, difficult to diagnostically separate from other conditions, and specific drugs for them are very expensive. My fairly strong instinct is that can't imagine this ever being a high impact area of intervention.
Could you explain a bit more? This doesn't seem entirely right, but you have more expertise here.
- It seems like they're relatively common. In Sharma et al., (2020) they say: "We examine two headache disorders: migraines and cluster headaches. The former are common, affecting around one in six people... Disability-Adjusted Life Years lost in 2019, according to the Global Burden of Disease, were 42 million for migraines, 46 million for malaria, and 47 million for depression (source)."
- I don't know anything about diagnosing migraines, so I'll trust you there.
- I get that specific migraine drugs are very expensive, but we specifically mentioned using NSAIDs, which seem relatively effective "Common NSAIDs can eliminate most pain from migraines in half of all cases: aspirin, 52% (Kirthi et al., 2013) or ibuprofen, 57% (Rabbie et al., 2013)."
I know these are only shallow investigations, but if you don't have access to experts on pain in LMIC countries (which probably exist, especially in the palliatve care field) I think you could potentially even save time early in your investigation process by talking to a few medical professionals in lower income countries to get more ideas and screen out a couple of these interventions.
Fair. Reaching out and talking to experts is not something we emphasized in these reports. The short calendar window for completing these reports made this difficult. Could we reach out to you if / when we look further into these topics?
NickLaing @ 2023-01-31T09:56 (+6)
Can always reach out to me!
Arthritis - same as treating most other pain - large amounts of paractamol, ibuprofen (and other nsaids) and diclofenac gel is what we do for arthritis.
I'm the opposite of an expert on migraines, and it looks like I'm technically just wrong. They are super common even in low income countries in the medical literature. After being here for 9 years I think I've only clearly diagnosed a migraine twice after seeing many thousands of patients. Maybe many with headaches (that we give ibuprofen to) might technically qualify as migraines but we just diagnose as tension headache. The migraine diagnosis is hardly made by anyone here.
Even with that I'm super dubious about that global burden of disease study which puts Migraine Dalys that nsanely high, but I don't know enough to specifically criticise.
What this means is that there would be multiple barriers to working on migraines - poor understanding by local clinicians (like me), teaching about correct treatment, then availability. Seems tricky and obviously local doctors here don't see it as a priority
Samuel Dupret @ 2023-01-31T11:00 (+2)
Hi Nick, A quick comment to thank you for engaging with our work and for your insights. This is super interesting.
Arthritis - same as treating most other pain - large amounts of paractamol, ibuprofen (and other nsaids) and diclofenac gel is what we do for arthritis.
This suggests that this could be really cost-effective, considering the price of NSAIDs! However, wouldn't issues of side effects also occur here? Or is this less of an issue because the gains would be higher?
NickLaing @ 2023-02-02T15:01 (+1)
Thanks Sam
Side effects issue is also there, but like I said it's more a thing to be aware of, that the intervention can't be too just spray ibuprofen into the masses (like is often done with malaria medication, ORS for diarrhoea etc.) because there is a relatively common, dangerous side effect. If it's given after asking the right questions and with good advice, it's no problem at all - in high income countries. we give out relatively large amounts of NSAIDs without being overly concerned
NickLaing @ 2023-02-01T05:30 (+1)
Preventing the side effects issue is more about good administration, and medications being given after good history taking and diagnosis by a clinician. In high income countries we have no qualms about giving l large amounts of nsaids, as long as we aren't giving them to people with gastritis, people are taking them with water and food and People understand what symptoms to look for to stop.
It just means it's a bad idea up have noon qualified prior giving them out like lollies, which does happen a lot. In many western countries nsaids are sold at supermarkets, which shows you it's not a game changing problem, just one to be careful about.
Rina @ 2023-01-27T16:51 (+5)
Super interesting read. Could I ask out of general curiosity how many hours of work were put into this? I know you've called it a shallow report and I see you point to areas where more digging can be done but this already has lots of depth too!
JoelMcGuire @ 2023-01-30T16:18 (+12)
Sam and I tracked a total of 143 hours for the research and writing of this report. We probably spent a bit more copy-editing and preparing for publication.
NickLaing @ 2023-01-30T17:36 (+3)
Wow it's insane that you produced something like this in so few hours. That's super impressive.
Rina @ 2023-01-31T17:04 (+1)
That's awesome! great work and thank you for sharing.
Erich_Grunewald @ 2023-01-27T18:03 (+2)
Nice work!
Ibuprofen is cheap. You can order 1,000 tablets online in Nigeria for $16. [...] As a result we estimate that the cost-effectiveness is 4.6 times GiveDirectly.
Looking at a couple of Nigerian online pharmacies (here, here), I see non-discount prices for Ibuprofen 400mg of 450 naira for 10 (I think) pills and 350 naira for 10 pills (generic brands, I think). At current exchange rates, 400 naira is $1.03, meaning 1,000 tablets would cost $103 excluding shipping, 6x your number. But maybe you can get them more cheaply buying in bulk?
NickLaing @ 2023-01-27T18:12 (+5)
I can tell you the exact cost of ibuprofen 200mg here in Uganda right now is about 8.50 for 1000 tablets, and it will be very similar in Nigeria, so the price may even be cheaper than the hli estimate. I have big questions about the morphine office though which I will reply to later.
Erich_Grunewald @ 2023-01-27T18:13 (+7)
Thanks! I guess the difference comes from buying in bulk, then.
jonleighton @ 2023-01-30T10:28 (+1)
Thanks for writing this report, which is really well researched!
One point I would stress, which you also hinted at, is the inadequacy of current metrics, including the WELLBY (though a definite improvement on the DALY and QALY), to properly account for the reality of extreme suffering. While 0-10 scales are common and useful, they impose the compression of a complex phenomenon onto a superficially linear scale that then gets treated as such, as if the different points on the scale represent equivalent increases in a cardinal unit. Pain and suffering that are so severe as to prompt suicidal ideations and attempts have a qualitatively distinct aspect that isn’t captured by such scales – probably not even if we interpreted them as being logarithmic. For this reason, reducing pain or suffering from a true 10 to an 8 is probably far more significant than reducing it from a 3 to a 1 – even if the timescale of the extreme suffering is much shorter.
For example, someone with cluster headaches might find the attacks on the verge of unbearability while experiencing them, similar to torture. A temporary drop in SWB during the attacks - even if it were to zero - wouldn’t sufficiently reflect the degree of agony. Furthermore, during attack-free periods, life satisfaction might be evaluated as relatively high and not reflect the suffering experienced during attacks.
Since interventions to reduce pain and other causes of suffering are ultimately aimed at reducing the phenomenon of suffering itself, there’s a strong case for new metrics to measure it more directly than the WELLBY does. In their 2017 paper, the Lancet Commission on Palliative Care and Pain Relief proposed the SALY (Suffering-Adjusted Life-Year). In my new book The Tango of Ethics, I suggested that the SALY could be reduced to YLS (Years Lived with Suffering), and I also proposed two additional metrics that could better account for the reality of severe and extreme suffering without being diluted by aggregation with moderate suffering: “Years Lived with Severe Suffering (YLSS) could capture suffering at the level of approximately 7/10 and above. A separate metric called Days Lived with Extreme Suffering (DLES) could capture the most urgent suffering at the level of approximately 9/10 and above, and properly account for it even when experienced on short timescales.” These metrics, and especially the latter, would ensure that the most severe suffering is not overlooked in public health interventions, and would allow us to better track states that have the highest urgency.
Of course, using additional metrics would complicate cost-effectiveness comparisons of interventions that have been evaluated in other terms, such as improved life satisfaction. But this would not be a valid reason not to use them, especially as parallel measures. If we want to address suffering so extreme that it causes people to kill themselves to escape it, we need to track it more directly.
A couple of specific points regarding interventions:
-Paying for the purchase of opioids could indeed help relieve the burden to healthcare systems in low-income countries in cases where palliative care and access to morphine are already a reality, but cost remains a barrier even after other obstacles have been lowered (logistics, training, regulatory…). Note that, because they are subject to international control, opioids in these countries are usually purchased centrally through the government and not by independent healthcare institutions.
-Advocacy for the legal provision of psychedelics to treat cluster headaches and some related conditions, which we are already engaged in, might be considered more cost-effective according to a metric that attributes particular importance to extreme pain/suffering, even if we can only provide a rough estimate of the impact of such campaigns.
(One minor point: it wasn't clear to me why going from 0 to 10 on a pain scale represents an 11-point change.)
JoelMcGuire @ 2023-01-30T20:18 (+10)
Hi Jon, I'm also concerned that subjective wellbeing measures may lose interpersonal comparability when someone experiences extreme suffering.
But it's not clear to me from the Lancet report or your description of the alternatives how we'd measure / construct them. Would it be like DALYs or QALYs? Would we ask the public to make time tradeoffs? Would we ask people with painful conditions to make tradeoffs or evaluate their experience?
I'm more open to alternatives here than I usually am, but I'd be very surprised if the best measure didn't ask the people with extreme suffering (or barring that, those close to them) about their experiences. This is because extreme suffering seems almost by definition something someone doesn't understand without having the misfortune to live with/despite it.
Thanks for the feedback on interventions! We will think about this when we do further work.
(One minor point: it wasn't clear to me why going from 0 to 10 on a pain scale represents an 11-point change.)
Oops! You're right. An 11-point scale (0 to 10) can only afford at most a 10 unit change.
jonleighton @ 2023-01-31T08:08 (+1)
Yes, I agree. Asking people who don't suffer from a condition to evaluate it is already an imprecise approach, and it just doesn't work for extreme suffering. I think self-evaluation is essential. A study on people with cluster headaches who had also experienced other sources of pain, including childbirth, kidney stones and gunshot wounds, provides a means of normalising the scale by re-setting the meaning of 10 (we reproduced the graph in our policy paper on cluster headaches). The measure was pain intensity, but the same approach could be used for suffering. Qualitative descriptors of different pain/suffering levels can help ensure that people mean the same thing in their self-evaluations. In my opinion, time tradeoffs can be helpful for ranking intensities of suffering, but I don't think they can substitute for direct intensity evaluations.