Scientists achieve record-breaking growth in miniature, functional liver models
By Connor Wood @ 2025-04-17T03:04 (+1)
At a Glance
Researchers have recently achieved a landmark million‑fold expansion of human hepatocyte organoids in just four weeks, while preserving vital liver functions such as bile acid transport, protein synthesis, and drug metabolism. Meanwhile, organ‑on‑chip systems replicate the liver’s microarchitecture — including sinusoidal networks — to evaluate toxicity under realistic flow conditions. Complementing these in vitro platforms, animal studies demonstrate how a plant‑based ketogenic diet reverses fibrosis in metabolic steatohepatitis (MASH), and electroacupuncture at specific acupoints mitigates antipsychotic‑induced lipid disturbances through liver–brain signaling. At the molecular level, epigenetic therapy corrects aberrant methylation in non‑alcoholic steatohepatitis (NASH), and probiotic interventions bolster liver health via the gut–liver axis. Together, these advances — from organoids and chips to diet, neural modulation, epigenetics, and microbiome strategies — offer comprehensive tools for modeling, understanding, and treating liver disease.
The Imperative for Human‑Relevant Liver Models
The liver plays a central role in metabolism, detoxification, immune regulation, and synthesis of critical biomolecules like clotting factors. When its functions fail, outcomes range from fatty liver and fibrosis to liver failure and cancer. Globally, one out of three adults shows signs of non‑alcoholic fatty liver disease (NAFLD), and deaths from liver conditions surpass two million annually. Traditional 2D cell cultures cannot mimic the liver’s 3D architecture, while rodent models differ in drug metabolism due to species variations in cytochrome P450 enzymes. A shift toward human‑relevant miniature liver models — organoids and organ‑on‑chip devices — addresses these gaps, offering higher predictive accuracy for drug safety and disease research.
In early drug screening, hepatocyte cultures often misestimate toxicity. When torcetrapib, a cholesterol‑lowering drug, advanced to clinical trials, unforeseen liver toxicity emerged in humans. Retrospective testing on liver‑on‑chips might have flagged this risk earlier by replicating human-specific metabolic pathways.
In Vivo Foundations: Rodent and Alternative Models
1.1 Ketogenic Diets in MASH Models
A vegetal oil–based ketogenic diet has shown promise in mouse models of metabolic dysfunction‑associated steatohepatitis (MASH). After eight weeks on a high‑cholesterol Western diet, mice exhibited ballooning hepatocytes and collagen deposition. Switching them to a ketogenic regimen enriched in coconut and olive oils led to:
- Reduced hepatic steatosis: Liver triglyceride content dropped by 40%.
- Attenuated inflammation: Fewer infiltrating macrophages were observed around fibrotic septa.
- Fibrosis reversal: Collagen fibers reshaped from thick bands to fine reticular networks.
Ketone bodies produced during nutritional ketosis can serve as alternative energy substrates, reducing reliance on glucose metabolism that drives lipogenesis. This metabolic shift alleviates lipid accumulation and oxidative stress in hepatocytes.
Patients with early-stage NAFLD who adopt a Mediterranean ketogenic diet may experience similar reductions in liver fat, though clinical trials are ongoing.
1.2 Electroacupuncture and Liver–Brain Crosstalk
Electroacupuncture at Zusanli (ST36) was applied for 20 minutes daily to mice treated with olanzapine, an antipsychotic known to induce weight gain and lipid dysregulation. Key outcomes included:
- Weight stabilization: Body weight gain slowed by 25% compared to controls.
- Normalization of free fatty acids (FFAs): Serum FFA levels returned to baseline.
- Improved hepatic lipid profile: Lower triglyceride content and normalized expression of PPARα and SREBP-1c.
Neural signals from ST36 travel via the vagus nerve, influencing hypothalamic centers that regulate appetite and peripheral lipid metabolism, illustrating a bidirectional liver–brain axis.
In clinical pilot studies, patients receiving adjunct acupuncture during antipsychotic treatment reported fewer metabolic side effects and better glycemic control.
Advanced In Vitro Platforms
2.1 Organ‑on‑Chip Technologies
Microfluidic liver‑on‑chip devices integrate:
- Sinusoidal channels: Narrow microchannels mimic blood flow patterns, creating shear stress that promotes endothelial‑hepatocyte interactions.
- 3D co‑culture zones: Hepatocytes, Kupffer cells, and stellate cells arranged in zonal fashions to replicate periportal-to-pericentral gradients.
- Sensor integration: Embedded electrodes monitor real‑time oxygen consumption and electrochemical markers.
Shear stress enhances cell polarization and function, while zonal gradients influence gene expression — some drugs exhibit zone-specific toxicity.
Troglitazone toxicity primarily affects the centrilobular region. Liver‑on‑chips reproducing this architecture correctly predicted troglitazone’s human-specific toxicity, whereas static cultures did not.
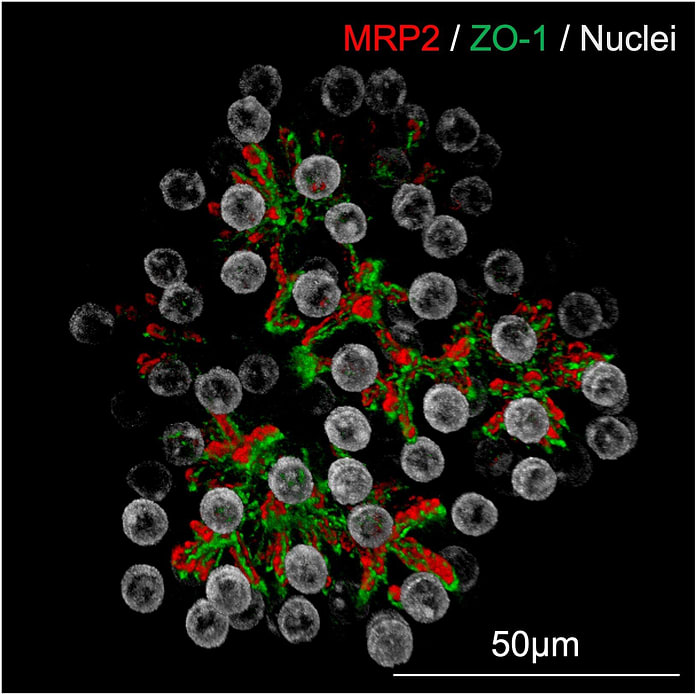
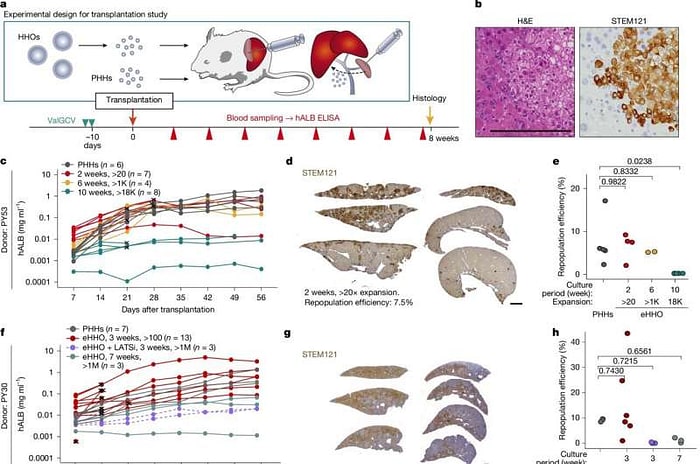
2.2 Miniature Liver Organoids with Record‑Breaking Growth
In a breakthrough, organoids derived from adult human hepatocytes exhibited:
- Million-fold expansion: Starting from 10,000 cells to over 10¹⁰ cells within four weeks.
- Stable phenotype: Expression of CYP3A4, albumin, and bile acid transporters remained at > 80% of primary hepatocytes.
- Transplant efficacy: When injected into Fah–/– mice (a model of liver failure), mice survived beyond 90 days with normal liver enzyme profiles.
A cocktail of Wnt agonists, EGF, and HGF supports self-renewal while preserving differentiation potential, enabling large-scale expansion.
Large organoid batches could enable batch‑wise drug screening panels with patient‑specific cells, reducing variability between experiments.
Molecular and Epigenetic Insights
3.1 Epigenetic Modulation in NASH
Pcyt2+/– mice develop NASH marked by global DNA hypermethylation. Treatment with phosphonoethylamine (PEA) resulted in:
- 90% reversal of hypermethylated CpG sites.
- Restoration of key metabolic genes, including PPARα and ADIPOQ, correlating with improved insulin sensitivity.
PEA serves as an alternative methyl donor that rebalances SAM/SAH ratios, normalizing DNA methyltransferase activity.
Epigenetic drugs could complement lifestyle interventions by reprogramming pathological gene expression patterns in NASH patients.
3.2 Stem Cell–Derived Organoids for DILI Screening
Human pluripotent stem cell–derived hepatic organoids (HLOs) have been optimized to include:
- CYP450 induction assays, detecting upregulation of CYP1A2, 2C9, and 3A4 upon drug exposure.
- Bile duct–like structures for cholestatic injury assessment.
DILI often arises from reactive metabolites formed by CYP enzymes. HLOs capture patient‑specific CYP variants, improving prediction of idiosyncratic toxicity.
In blinded panels, HLOs correctly classified 95% of known hepatotoxic drugs, outperforming HepG2 cell lines.
Lipid Homeostasis: The Role of LFABP
Immunofluorescence assays using Bodipy‑labeled fatty acids revealed:
- LFABP colocalizes with ApoB-containing vesicles in the Golgi, driving VLDL assembly.
- LFABP knockdown reduces VLDL secretion by 60%, leading to intracellular lipid droplet accumulation.
LFABP buffers free fatty acids, directing them to appropriate metabolic or secretory pathways, critical for maintaining plasma lipid homeostasis.
Genetic variants in the FABP1 gene correlate with altered plasma triglyceride levels in human cohorts.
Therapeutic Horizons: Gut–Liver Axis and Probiotics
In high-fat‑diet mouse models, Lactiplantibacillus plantarum Lb41:
- Restores gut barrier integrity, reducing endotoxin translocation to the liver.
- Adjusts bile acid profiles, increasing secondary bile acids that activate hepatic FXR receptors to limit lipogenesis.
- Downregulates TNF-α and IL-6 expression in the liver, lowering inflammation.
The gut microbiota modulates bile acid metabolism and immune signaling, directly impacting hepatic steatosis and fibrosis.
Pilot human trials using probiotic cocktails show promising reductions in liver fat content measured by MRI‑PDFF.
Key Takeaways for Beginners
- Choose wisely: Match your model to the question — dietary, molecular, toxicity, or regenerative medicine.
- Combine approaches: In vitro organoids for mechanistic studies, organ‑on‑chips for toxicity screening, and in vivo models for systemic effects.
- Scale matters: Scalable organoid expansion and microfluidic chips bring clinical translation within reach.
- Integrate systems: Diet, neural, epigenetic, and microbiome interventions converge on liver health.
Future Directions
- Bioreactors for Organoids: Automated perfusion systems to produce clinical‑grade hepatocyte batches.
- Immune‑Competent Chips: Integrating Kupffer and lymphocyte populations for immunotoxicology.
- Multi‑Organ Platforms: Connecting liver chips with gut, kidney, or brain modules to study organ crosstalk.
- Personalized Medicine: Patient-specific organoid panels for tailoring drug dosages and predicting adverse reactions.
Meet Pubmed.ai
Pubmed.ai is an innovative research tool that harnesses the power of artificial intelligence to transform how clinicians, researchers, and students access medical literature. Connected directly to the extensive PubMed database, Pubmed.ai allows users to simply enter a keyword — whether it’s a specific disease, condition, or broader medical topic — and instantly retrieve a curated list of relevant articles. The system goes beyond mere retrieval; it automatically generates a concise introduction to the topic and extracts the key takeaways from the available literature, enabling users to quickly grasp the essence of the research. This blog was written with the help of pubmed.ai, which can help scholars and researchers search for resource information more smoothly on PubMed. It can even generate a complete academic report for you. Whether you are looking to stay updated on the latest advances or conducting in-depth academic research, Pubmed.ai offers a forward-thinking solution that simplifies the navigation of vast biomedical resources. Embrace the future of medical research with Pubmed.ai, where a single keyword unlocks a world of knowledge and expertly distilled information at your fingertips.
References
Al Reza, H., Santangelo, C., Iwasawa, K., Al Reza, A., Sekiya, S., Glaser, K., Bondoc, A., Merola, J., & Takebe, T. (2025). Multi‑zonal liver organoids from human pluripotent stem cells. Nature. Advance online publication. https://doi.org/10.1038/s41586-025-08850-1
Chen, J., Ju, P., Luo, C., Tu, S., Sun, Y., Shi, S., Sun, G., Huang, L., Wang, Z., Xu, Y., Shi, Y., & Wu, H. (2025). Effect of electroacupuncture at Zusanli (ST36) on olanzapine‑induced lipid disturbances in mice via potential liver–brain interaction. The FASEB Journal, 39(8), e70491. https://doi.org/10.1096/fj.202402319R
Fu, J., Qiu, H., & Tan, C. S. (2023). Microfluidic liver‑on‑a‑chip for preclinical drug discovery. Pharmaceutics, 15(4), 1300. https://doi.org/10.3390/pharmaceutics15041300
Grapentine, S., Agarwal, P., Dolinsky, V. W., & Bakovic, M. (2025). Epigenome‑wide methylation analysis shows phosphonoethylamine alleviates aberrant DNA methylation in NASH caused by Pcyt2 deficiency. PLoS One, 20(3), e0320510. https://doi.org/10.1371/journal.pone.0320510
Igarashi, R., Oda, M., Okada, R., Yano, T., Takahashi, S., Pastuhov, S., Matano, M., Masuda, N., Togasaki, K., Ohta, Y., Sato, S., Hishiki, T., Suematsu, M., Itoh, M., Fujii, M., & Sato, T. (2025). Generation of human adult hepatocyte organoids with metabolic functions. Nature. Advance online publication. https://doi.org/10.1038/s41586-025-08861-y
Kim, H., Kim, S. K., Oelgeschläger, M., & Park, H.-J. (2024). Prediction of acute hepatotoxicity with human pluripotent stem cell‑derived hepatic organoids. Current Protocols, 4(4), e1015. https://doi.org/10.1002/cpz1.1015
Lee, N.-K., Lee, Y., Shin, D.-S., Park, E., & Paik, H.-D. (2025). Probiotic Lactiplantibacillus plantarum Lb41 alleviates high‑fat diet‑induced nonalcoholic fatty liver disease in mice. Nutrition, 134, 112735. https://doi.org/10.1016/j.nut.2025.112735
Provera, A., Ramavath, N. N., Gadipudi, L. L., Vecchio, C., Caputo, M., Antonioli, A., Tini, S., Sheferaw, A. N., Reano, S., Filigheddu, N., Manfredi, M., Barberis, E., Cocolin, L., Ferrocino, I., Locatelli, M., Caprio, M., Tacke, F., Albano, E., Prodam, F., & Sutti, S. (2025). Vegetal oil‑based ketogenic diet improves inflammation and fibrosis in experimental metabolic dysfunction‑associated steatohepatitis. Frontiers in Immunology, 16, 1518687. https://doi.org/10.3389/fimmu.2025.1518687
Winterfeldt, K., Tasin, F. R., & Siddiqi, S. A. (2025). Establishing the role of liver fatty acid‑binding protein in post‑Golgi very‑low‑density lipoprotein trafficking using a novel fluorescence‑based assay. International Journal of Molecular Sciences, 26(6), 2399. https://doi.org/10.3390/ijms26062399
Al Reza, H., Santangelo, C., Iwasawa, K., Al Reza, A., Sekiya, S., Glaser, K., Bondoc, A., Merola, J., & Takebe, T. (2025). Multi‑zonal liver organoids from human pluripotent stem cells. Nature. Advance online publication. https://doi.org/10.1038/s41586-025-08850-1